Gut Metabolite Trimethylamine N-Oxide Protects INS-1 β-Cell and Rat Islet Function under Diabetic Glucolipotoxic Conditions
- PMID: 34944536
- PMCID: PMC8699500
- DOI: 10.3390/biom11121892
Gut Metabolite Trimethylamine N-Oxide Protects INS-1 β-Cell and Rat Islet Function under Diabetic Glucolipotoxic Conditions
Abstract
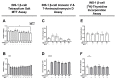
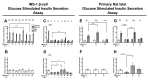
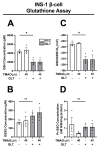
Serum accumulation of the gut microbial metabolite trimethylamine N-oxide (TMAO) is associated with high caloric intake and type 2 diabetes (T2D). Impaired pancreatic β-cell function is a hallmark of diet-induced T2D, which is linked to hyperglycemia and hyperlipidemia. While TMAO production via the gut microbiome-liver axis is well defined, its molecular effects on metabolic tissues are unclear, since studies in various tissues show deleterious and beneficial TMAO effects. We investigated the molecular effects of TMAO on functional β-cell mass. We hypothesized that TMAO may damage functional β-cell mass by inhibiting β-cell viability, survival, proliferation, or function to promote T2D pathogenesis. We treated INS-1 832/13 β-cells and primary rat islets with physiological TMAO concentrations and compared functional β-cell mass under healthy standard cell culture (SCC) and T2D-like glucolipotoxic (GLT) conditions. GLT significantly impeded β-cell mass and function by inducing oxidative and endoplasmic reticulum (ER) stress. TMAO normalized GLT-mediated damage in β-cells and primary islet function. Acute 40µM TMAO recovered insulin production, insulin granule formation, and insulin secretion by upregulating the IRE1α unfolded protein response to GLT-induced ER and oxidative stress. These novel results demonstrate that TMAO protects β-cell function and suggest that TMAO may play a beneficial molecular role in diet-induced T2D conditions.
Keywords: beta cell; glucolipotoxicity (GLT); glucose stimulated insulin secretion (GSIS); islet; type 2 diabetes (T2D); unfolded protein response (UPR).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Stanhope K.L., Goran M.I., Bosy-Westphal A., King J.C., Schmidt L.A., Schwarz J.M., Stice E., Sylvetsky A.C., Turnbaugh P.J., Bray G.A., et al. Pathways and mechanisms linking dietary components to cardiometabolic disease: Thinking beyond calories. Obes. Rev. 2018;19:1205–1235. doi: 10.1111/obr.12699. - DOI - PMC - PubMed
-
- Svingen G.F., Schartum-Hansen H., Pedersen E.R., Ueland P.M., Tell G.S., Mellgren G., Njolstad P.R., Seifert R., Strand E., Karlsson T., et al. Prospective Associations of Systemic and Urinary Choline Metabolites with Incident Type 2 Diabetes. Clin. Chem. 2016;62:755–765. doi: 10.1373/clinchem.2015.250761. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

