Combined Therapy of Vitamin D3-Tolerogenic Dendritic Cells and Interferon-β in a Preclinical Model of Multiple Sclerosis
- PMID: 34944573
- PMCID: PMC8698295
- DOI: 10.3390/biomedicines9121758
Combined Therapy of Vitamin D3-Tolerogenic Dendritic Cells and Interferon-β in a Preclinical Model of Multiple Sclerosis
Abstract
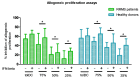
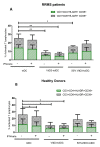
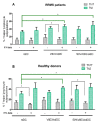
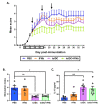
Autologous antigen-specific therapies based on tolerogenic dendritic cells (tolDC) offer the possibility to treat autoimmune diseases by restoring homeostasis and targeting specifically autoreactive responses. Here, we explore the hypothesis that systemic inflammation occurring in autoimmune diseases, such as multiple sclerosis (MS), can generate a disease-specific environment able to alter the functionality of tolDC. In this context in fact, a combined therapy of tolDC with an immunomodulatory treatment could potentiate the beneficial effect of this antigen-specific cell therapy. For this purpose, we analyzed the efficacy of a combined therapy based on the use of vitamin D3 (VitD3)-tolDC plus interferon beta (IFN-beta) in MS. VitD3-tolDC were generated from healthy donors and MS patients and co-cultured with allogeneic peripheral blood mononuclear cells, in the presence or absence of IFN-beta. In vitro, VitD3-tolDC treatment reduced the percentage of activated T cells and allogeneic proliferation, whereas VitD3-tolDC+IFN-beta treatment enhanced the suppressive ability of VitD3-tolDC and, additionally, induced a shift towards a Th2 profile. To determine the clinical benefit of the combined therapy, C57BL/6-experimental autoimmune encephalomyelitis (EAE)-induced mice were treated with antigen-specific VitD3-tolDC and/or IFN-beta. Treatment of EAE mice with combined therapy ameliorated the disease course compared to each monotherapy. These results suggest that a combined therapy based on antigen-specific VitD3-tolDC and IFN-beta may represent a promising strategy for MS patients.
Keywords: antigen-specific therapies; combined therapy; immunomodulatory; multiple sclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mansilla M.J., Presas-Rodríguez S., Teniente-Serra A., González-Larreategui I., Quirant-Sánchez B., Fondelli F., Djedovic N., Iwaszkiewicz-Grześ D., Chwojnicki K., Miljković Đ., et al. Paving the way towards an effective treatment for multiple sclerosis: Advances in cell therapy. Cell. Mol. Immunol. 2021;18:1353–1374. doi: 10.1038/s41423-020-00618-z. - DOI - PMC - PubMed
-
- Machado-Santos J., Saji E., Tröscher A., Paunovic M., Liblau R., Gabriely G., Bien C.G., Bauer J., Lassmann H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141:2066–2082. doi: 10.1093/brain/awy151. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

