Lateralized and Segmental Overgrowth in Children
- PMID: 34944785
- PMCID: PMC8699773
- DOI: 10.3390/cancers13246166
Lateralized and Segmental Overgrowth in Children
Abstract
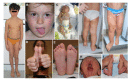
Congenital disorders of lateralized or segmental overgrowth (LO) are heterogeneous conditions with increased tissue growth in a body region. LO can affect every region, be localized or extensive, involve one or several embryonic tissues, showing variable severity, from mild forms with minor body asymmetry to severe ones with progressive tissue growth and related relevant complications. Recently, next-generation sequencing approaches have increased the knowledge on the molecular defects in LO, allowing classifying them based on the deranged cellular signaling pathway. LO is caused by either genetic or epigenetic somatic anomalies affecting cell proliferation. Most LOs are classifiable in the Beckwith-Wiedemann spectrum (BWSp), PI3KCA/AKT-related overgrowth spectrum (PROS/AROS), mosaic RASopathies, PTEN Hamartoma Tumor Syndrome, mosaic activating variants in angiogenesis pathways, and isolated LO (ILO). These disorders overlap over common phenotypes, making their appraisal and distinction challenging. The latter is crucial, as specific management strategies are key: some LO is associated with increased cancer risk making imperative tumor screening since childhood. Interestingly, some LO shares molecular mechanisms with cancer: recent advances in tumor biological pathway druggability and growth downregulation offer new avenues for the treatment of the most severe and complicated LO.
Keywords: Beckwith–Wiedemann; PIK3CA; PROS; cancer predisposition; cancer screening; lateralized; mosaic; overgrowth; segmental; somatic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kalish J.M., Biesecker L.G., Brioude F., Deardorff M.A., Di Cesare-Merlone A., Druley T., Ferrero G.B., Lapunzina P., Larizza L., Maas S., et al. Nomenclature and definition in asymmetric regional body overgrowth. Am. J. Med. Genet. A. 2017;173:1735–1738. doi: 10.1002/ajmg.a.38266. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

