Characterization of Genetic Heterogeneity in Recurrent Metastases of Renal Cell Carcinoma
- PMID: 34944839
- PMCID: PMC8699544
- DOI: 10.3390/cancers13246221
Characterization of Genetic Heterogeneity in Recurrent Metastases of Renal Cell Carcinoma
Abstract
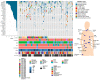
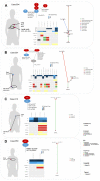
Metastatic renal cell carcinoma (RCC) exhibits poor prognosis. Better knowledge of distant metastases is crucial to foster personalized treatment strategies. Here, we aimed to investigate the genetic landscape of metastases, including synchronous and/or recurrent metastases to elucidate potential drug target genes and clinically relevant mutations in a real-world setting of patients. We assessed 81 metastases from 56 RCC patients, including synchronous and/or recurrent metastases of 19 patients. Samples were analysed through next-generation sequencing with a high coverage (~1000× mean coverage). We therefore established a novel sequencing panel comprising 32 genes with impact on RCC development. We observed a high frequency of mutations in known RCC driver genes (e.g., >40% carriers of VHL and PBRM1 mutations) in metastases irrespective of the metastatic site. The somatic mutational composition was significantly associated with cancer-specific survival (p(logrank) = 0.03). Moreover, we identified in 34 patients at least one drug target gene as well as clinically relevant mutations listed in the VICC Meta-Knowledgebase in 7%. In addition to significantly higher mutational burden in recurrent metastases compared to earlier ones, synchronous and/or recurrent metastases of individual patients, even after a time-period >2 yrs, shared a high proportion of somatic events. Our data demonstrate the importance of somatic profiling in metastases for precision medicine in RCC.
Keywords: metastasis; next-generation sequencing; personalized therapy; pharmacogenomics; renal cell carcinoma.
Conflict of interest statement
J.B.: Personal honoraria for speaker, consultancy, or advisory role: AstraZeneca, Astellas, BMS, Eisai, Ipsen, MSD, Novartis, Roche, EUSA Pharma, Nektar, Pfizer; Institutional financial interests which have been paid directly to your institution: Eisai, Ipsen, MSD, Novartis, Roche, Pfizer. A.S.: consultancies, honoraria, or study participation from Bayer, BMS, Immatics, Novartis, Pfizer, and Roche. S.R.: honoraria for speaker, advisory role: Astellas, Bayer, Pfizer, and Merck. The remaining authors have nothing to disclose. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Bedke J., Albiges L., Capitanio U., Giles R.H., Hora M., Lam T.B., Ljungberg B., Marconi L., Klatte T., Volpe A., et al. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Nivolumab plus Cabozantinib Joins Immune Checkpoint Inhibition Combination Therapies for Treatment-naïve Metastatic Clear-Cell Renal Cell Carcinoma. Eur. Urol. 2021:339–342. doi: 10.1016/j.eururo.2020.12.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

