Interstitial High-Dose-Rate Brachytherapy of Liver Metastases in Oligometastatic Patients
- PMID: 34944869
- PMCID: PMC8699459
- DOI: 10.3390/cancers13246250
Interstitial High-Dose-Rate Brachytherapy of Liver Metastases in Oligometastatic Patients
Abstract
Local ablative treatments have emerged as a promising treatment strategy for patients with oligometastatic disease. Among others, interstitial brachytherapy (iBT) is an upcoming treatment option for unresectable liver metastases. We report the feasibility and oncologic outcome of iBT of oligometastatic liver metastases performed in patients with limited tumor burdens in a high-volume center. Patients undergoing iBT between August 2017and March 2019 were included. A retrospective analysis of patient outcomes and treatment complications was performed. Patients treated for metastatic colorectal carcinoma (CRC) were compared to other histologies. A total of 141 iBT procedures were performed in 106 patients (male:52; female:54) and 244 liver metastases. Overall, 51% (54/106) of patients had a diagnosis of metastatic CRC. The median follow-up was 9 months, and overall survival (OS) was 92.3% at 6 months and 76.3% at 12 months. Local-relapse-free survival (LRFS) was 88.4% at 6 months and 71.5% at 12 months, with a significant difference between patients with CRC (84.1% and 50.6%) versus other histologies (92.4% and 92.4%, p < 0.001). A sub-group analysis showed a significant advantage in patients with CRC receiving a minimal dose (D100) of 20 Gy to the planning target volume. Treatments of smaller total liver-tumor volumes (<18 ccm) resulted in better LRFS rates. iBT is a safe and effective treatment approach for oligometastatic liver disease. A higher treatment dose is needed for patients with CRC. Moreover, lower metastatic burdens may be favorable for LRFS. Prospective studies are needed to assess the role of iBT in the oligometastatic setting as an alternative to other local ablative treatment approaches in patients with liver metastases.
Keywords: brachytherapy; liver; local control; metastases; outcome; radiotherapy; survival.
Conflict of interest statement
C.B., G.L. and S.C. have received research grants from Elekta. S.C. and C.B. have received speaker honoraria/travel support from Elekta. The other authors declare no conflict of interest.
Figures
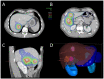
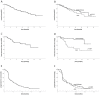
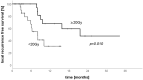
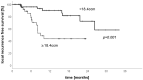
References
-
- Guckenberger M., Lievens Y., Bouma A.B., Collette L., Dekker A., Desouza N.M., Dingemans A.-M.C., Fournier B., Hurkmans C., Lecouvet F.E., et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–e28. doi: 10.1016/S1470-2045(19)30718-1. - DOI - PubMed
-
- Lievens Y., Guckenberger M., Gomez D., Hoyer M., Iyengar P., Kindts I., Romero A.M., Nevens D., Palma D., Park C., et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. - DOI - PubMed
-
- Ost P., Reynders D., Decaestecker K., Fonteyne V., Lumen N., De Bruycker A., Lambert B., Delrue L., Bultijnck R., Claeys T., et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. - DOI - PubMed
-
- Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., Mulroy L., Lock M., Rodrigues P.G.B., Yaremko B.P., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources

