The Transmembrane Receptor TIRC7 Identifies a Distinct Subset of Immune Cells with Prognostic Implications in Cholangiocarcinoma
- PMID: 34944891
- PMCID: PMC8699724
- DOI: 10.3390/cancers13246272
The Transmembrane Receptor TIRC7 Identifies a Distinct Subset of Immune Cells with Prognostic Implications in Cholangiocarcinoma
Abstract
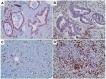
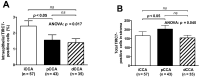
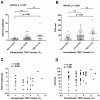
Cholangiocarcinoma (CCA) is a heterogeneous malignancy with a dismal prognosis. Therapeutic options are largely limited to surgery and conventional chemotherapy offers limited benefit. As immunotherapy has proven highly effective in various cancer types, we have undertaken a quantitative immunohistopathological assessment of immune cells expressing the immunoinhibitory T cell immune response cDNA 7 receptor (TIRC7), an emerging immunoinhibitory receptor, in a cohort of 135 CCA patients. TIRC7+ immune cells were present in both the tumor epithelia and stroma in the majority of CCA cases with the highest levels found in intrahepatic CCA. While intraepithelial density of TIRC7+ immune cells was decreased compared to matched non-neoplastic bile ducts, stromal quantity was higher in the tumor samples. Tumors exhibiting signet ring cell or adenosquamous morphology were exclusively associated with an intraepithelial TIRC7+ phenotype. Survival analysis showed intraepithelial TIRC7+ immune cell density to be a highly significant favorable prognosticator in intrahepatic but not proximal or distal CCA. Furthermore, intraepithelial TIRC7+ immune cell density correlated with the number of intraepithelial CD8+ immune cells and with the total number of CD4+ immune cells. Our results suggest the presence and prognostic relevance of TIRC7+ immune cells in CCA and warrant further functional studies on its pharmacological modulation.
Keywords: PD-L1; TIRC7; biomarkers; cholangiocarcinoma; immune checkpoint inhibitors; immunotherapy; tumor microenvironment.
Conflict of interest statement
P.S. received funding for grants, boards, and presentations from Novartis. The other authors declare no competing interests.
Figures


Similar articles
-
Inhibition of T-cell-mediated immune response via the PD-1/ PD-L1 axis in cholangiocarcinoma cells.Eur J Pharmacol. 2021 Apr 15;897:173960. doi: 10.1016/j.ejphar.2021.173960. Epub 2021 Feb 19. Eur J Pharmacol. 2021. PMID: 33617828
-
Immunotherapy of cholangiocarcinoma: Therapeutic strategies and predictive biomarkers.Cancer Lett. 2022 Oct 10;546:215853. doi: 10.1016/j.canlet.2022.215853. Epub 2022 Jul 31. Cancer Lett. 2022. PMID: 35921970 Review.
-
HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1.J Immunother Cancer. 2019 Mar 18;7(1):77. doi: 10.1186/s40425-019-0554-8. J Immunother Cancer. 2019. PMID: 30885276 Free PMC article.
-
Immunosuppressive tumor microenvironment in occupational cholangiocarcinoma: Supportive evidence for the efficacy of immune checkpoint inhibitor therapy.J Hepatobiliary Pancreat Sci. 2020 Nov;27(11):860-869. doi: 10.1002/jhbp.788. Epub 2020 Jul 20. J Hepatobiliary Pancreat Sci. 2020. PMID: 32506715
-
The immune milieu of cholangiocarcinoma: From molecular pathogenesis to precision medicine.J Autoimmun. 2019 Jun;100:17-26. doi: 10.1016/j.jaut.2019.03.007. Epub 2019 Mar 9. J Autoimmun. 2019. PMID: 30862450 Review.
Cited by
-
Socioeconomic disparities in endometrial cancer survival in Germany: a survival analysis using population-based cancer registry data.J Cancer Res Clin Oncol. 2022 May;148(5):1087-1095. doi: 10.1007/s00432-021-03908-9. Epub 2022 Jan 22. J Cancer Res Clin Oncol. 2022. PMID: 35064816 Free PMC article.
References
-
- Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., Cardinale V., Carpino G., Andersen J.B., Braconi C., et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

