Orai1 Boosts SK3 Channel Activation
- PMID: 34944977
- PMCID: PMC8699475
- DOI: 10.3390/cancers13246357
Orai1 Boosts SK3 Channel Activation
Abstract
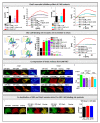
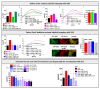
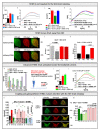
The interplay of SK3, a Ca2+ sensitive K+ ion channel, with Orai1, a Ca2+ ion channel, has been reported to increase cytosolic Ca2+ levels, thereby triggering proliferation of breast and colon cancer cells, although a molecular mechanism has remained elusive to date. We show in the current study, via heterologous protein expression, that Orai1 can enhance SK3 K+ currents, in addition to constitutively bound calmodulin (CaM). At low cytosolic Ca2+ levels that decrease SK3 K+ permeation, co-expressed Orai1 potentiates SK3 currents. This positive feedback mechanism of SK3 and Orai1 is enabled by their close co-localization. Remarkably, we discovered that loss of SK3 channel activity due to overexpressed CaM mutants could be restored by Orai1, likely via its interplay with the SK3-CaM binding site. Mapping for interaction sites within Orai1, we identified that the cytosolic strands and pore residues are critical for a functional communication with SK3. Moreover, STIM1 has a bimodal role in SK3-Orai1 regulation. Under physiological ionic conditions, STIM1 is able to impede SK3-Orai1 interplay by significantly decreasing their co-localization. Forced STIM1-Orai1 activity and associated Ca2+ influx promote SK3 K+ currents. The dynamic regulation of Orai1 to boost endogenous SK3 channels was also determined in the human prostate cancer cell line LNCaP.
Keywords: CRAC channel; Ca2+ activated K+ ion channels; LNCaP cells; Orai1; SK channels; SK3; STIM1; calmodulin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Jardin I., Diez-Bello R., Lopez J.J., Redondo P.C., Salido G.M., Smani T., Rosado J.A. TRPC6 Channels Are Required for Proliferation, Migration and Invasion of Breast Cancer Cell Lines by Modulation of Orai1 and Orai3 Surface Exposure. Cancers. 2018;10:331. doi: 10.3390/cancers10090331. - DOI - PMC - PubMed
-
- Rosado J.A. Calcium Entry Pathways in Non-excitable Cells. Preface. Adv. Exp. Med. Biol. 2016;898:VII–VIII. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

