D-Mannose Slows Glioma Growth by Modulating Myeloperoxidase Activity
- PMID: 34944979
- PMCID: PMC8699108
- DOI: 10.3390/cancers13246360
D-Mannose Slows Glioma Growth by Modulating Myeloperoxidase Activity
Abstract
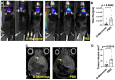
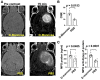
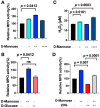
Host immune response in the tumor microenvironment plays key roles in tumorigenesis. We hypothesized that D-mannose, a simple sugar with anti-inflammatory properties, could decrease oxidative stress and slow glioma progression. Using a glioma stem cell model in immunocompetent mice, we induced gliomas in the brain and tracked MPO activity in vivo with and without D-mannose treatment. As expected, we found that D-mannose treatment decreased the number of MPO+ cells and slowed glioma progression compared to PBS-treated control animals with gliomas. Unexpectedly, instead of decreasing MPO activity, D-mannose increased MPO activity in vivo, revealing that D-mannose boosted the MPO activity per MPO+ cell. On the other hand, D-glucose had no effect on MPO activity. To better understand this effect, we examined the effect of D-mannose on bone marrow-derived myeloid cells. We found that D-mannose modulated MPO activity via two mechanisms: directly via N-glycosylation of MPO, which boosted the MPO activity of each molecule, and indirectly by increasing H2O2 production, the main substrate for MPO. This increased host immune response acted to reduce tumor size, suggesting that increasing MPO activity such as through D-mannose administration may be a potential new therapeutic direction for glioma treatment.
Keywords: D-mannose; MPO activity; glioma; leukocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

