Predictive Biomarkers for Outcomes of Immune Checkpoint Inhibitors (ICIs) in Melanoma: A Systematic Review
- PMID: 34944986
- PMCID: PMC8699321
- DOI: 10.3390/cancers13246366
Predictive Biomarkers for Outcomes of Immune Checkpoint Inhibitors (ICIs) in Melanoma: A Systematic Review
Abstract
Immune checkpoint inhibitors (ICIs) have strongly improved the survival of melanoma patients. However, as durable response to ICIs are only seen in a minority, there is an unmet need to identify biomarkers that predict response. Therefore, we provide a systematic review that evaluates all biomarkers studied in association with outcomes of melanoma patients receiving ICIs. We searched Pubmed, COCHRANE Library, Embase, Emcare, and Web of Science for relevant articles that were published before June 2020 and studied blood, tumor, or fecal biomarkers that predicted response or survival in melanoma patients treated with ICIs. Of the 2536 identified reports, 177 were included in our review. Risk of bias was high in 40%, moderate in 50% and low in 10% of all studies. Biomarkers that correlated with response were myeloid-derived suppressor cells (MDSCs), circulating tumor cells (CTCs), CD8+ memory T-cells, T-cell receptor (TCR) diversity, tumor-infiltrating lymphocytes (TILs), gene expression profiling (GEP), and a favorable gut microbiome. This review shows that biomarkers for ICIs in melanoma patients are widely studied, but heterogeneity between studies is high, average sample sizes are low, and validation is often lacking. Future studies are needed to further investigate the predictive utility of some promising candidate biomarkers.
Keywords: biomarkers; immune checkpoint inhibitor; melanoma; prediction; response; systematic review.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
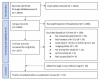
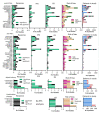

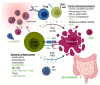
References
-
- Integraal Kankercentrum Nederland Cijfers over Kanker. [(accessed on 23 July 2021)]. Available online: www.cijfersoverkanker.nl.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

