Anti-SARS-CoV-2 S-RBD IgG Antibody Responses after COVID-19 mRNA Vaccine in the Chronic Disorder of Consciousness: A Pilot Study
- PMID: 34945125
- PMCID: PMC8708169
- DOI: 10.3390/jcm10245830
Anti-SARS-CoV-2 S-RBD IgG Antibody Responses after COVID-19 mRNA Vaccine in the Chronic Disorder of Consciousness: A Pilot Study
Abstract
Objective: In the last year, a large amount of research has investigated the anti-spike protein receptor-binding domain (S-RBD) antibody responses in patients at high risk of developing severe acute respiratory syndrome because of COVID-19 infection. However, no data are available on the chronic disorder of consciousness (DOC).
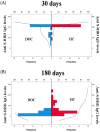
Methods: Here, we evaluated anti-S-RBD IgG levels after vaccination in chronic DOC patients compared with demographically matched healthy controls (HC) by indirect chemiluminescence immunoassay. All individuals completed a two-dose-cycle vaccination with Pfizer mRNA vaccine (BNT162b2), and antibody responses were evaluated at 30 and 180 days after the administration of the second dose of vaccination.
Results: We compared 32 DOC patients with 34 demographically matched healthy controls. Both DOC and HC groups showed a similar antibody response at 30 days, whereas at follow-up (180 days) DOC patients were characterized by lower S-RBD IgG levels with respect to controls. Additional multiple regression analyses including demographical and clinical comorbidities as predictors revealed that age was the only factor associated with the decrease in S-RBD IgG levels at follow-up (180 days). Elderly individuals of both groups were characterized by a reduction in the antibody responses with respect to younger individuals.
Conclusions: Our results show an efficacy seroconversion in DOC patients in the first period after vaccination, which significantly declines over time with respect to healthy controls.
Keywords: COVID-19; antibody responses; disorder of consciousness; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization (WHO) Novel Coronavirus (2019-nCoV) Situation Report—1 21 January 2020. WHO; Geneva, Switzerland: 2020. [(accessed on 29 January 2020)]. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. Erratum in 2020, 8, e26. - DOI - PMC - PubMed
-
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

