Microfluidic Cell Transport with Piezoelectric Micro Diaphragm Pumps
- PMID: 34945309
- PMCID: PMC8708163
- DOI: 10.3390/mi12121459
Microfluidic Cell Transport with Piezoelectric Micro Diaphragm Pumps
Abstract
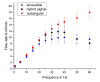
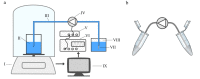
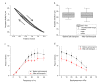
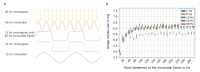
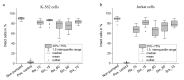
The automated transport of cells can enable far-reaching cell culture research. However, to date, such automated transport has been achieved with large pump systems that often come with long fluidic connections and a large power consumption. Improvement is possible with space- and energy-efficient piezoelectric micro diaphragm pumps, though a precondition for a successful use is to enable transport with little to no mechanical stress on the cell suspension. This study evaluates the impact of the microfluidic transport of cells with the piezoelectric micro diaphragm pump developed by our group. It includes the investigation of different actuation signals. Therewith, we aim to achieve optimal fluidic performance while maximizing the cell viability. The investigation of fluidic properties proves a similar performance with a hybrid actuation signal that is a rectangular waveform with sinusoidal flanks, compared to the fluidically optimal rectangular actuation. The comparison of the cell transport with three actuation signals, sinusoidal, rectangular, and hybrid actuation shows that the hybrid actuation causes less damage than the rectangular actuation. With a 5% reduction of the cell viability it causes similar strain to the transport with sinusoidal actuation. Piezoelectric micro diaphragm pumps with the fluidically efficient hybrid signal actuation are therefore an interesting option for integrable microfluidic workflows.
Keywords: automated cell culture; cell transport; micro diaphragm pump; micro dosing; microfluidic; passive spring valves.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Kurniawan Y.S. Micro Total Analysis System Application for Biomedicals: A Mini-Review. Biomed. J. Sci. Tech. Res. 2019;12:1–2. doi: 10.26717/BJSTR.2019.12.002294. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

