Benchmarking Outcomes after Ablative Radiotherapy for Molecularly Characterized Intrahepatic Cholangiocarcinoma
- PMID: 34945742
- PMCID: PMC8703854
- DOI: 10.3390/jpm11121270
Benchmarking Outcomes after Ablative Radiotherapy for Molecularly Characterized Intrahepatic Cholangiocarcinoma
Abstract
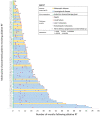
We have previously shown that ablative radiotherapy (A-RT) with a biologically effective dose (BED10) ≥ 80.5 Gy for patients with unresectable intrahepatic cholangiocarcinoma (ICC) is associated with longer survival. Despite recent large-scale sequencing efforts in ICC, outcomes following RT based on genetic alterations have not been described. We reviewed records of 156 consecutive patients treated with A-RT for unresectable ICC from 2008 to 2020. For 114 patients (73%), next-generation sequencing provided molecular profiles. The overall survival (OS), local control (LC), and distant metastasis-free survival (DMFS) were estimated using the Kaplan-Meier method. Univariate and multivariable Cox analyses were used to determine the associations with the outcomes. The median tumor size was 7.3 (range: 2.2-18.2) cm. The portal vein thrombus (PVT) was present in 10%. The RT median BED10 was 98 Gy (range: 81-144 Gy). The median (95% confidence interval) follow-up was 58 (42-104) months from diagnosis and 39 (33-74) months from RT. The median OS was 32 (29-35) months after diagnosis and 20 (16-24) months after RT. The one-year OS, LC, and intrahepatic DMFS were 73% (65-80%), 81% (73-87%), and 34% (26-42%). The most common mutations were in IDH1 (25%), TP53 (22%), ARID1A (19%), and FGFR2 (13%). Upon multivariable analysis, the factors associated with death included worse performance status, larger tumor, metastatic disease, higher CA 19-9, PVT, satellitosis, and IDH1 and PIK3CA mutations. TP53 mutation was associated with local failure. Further investigation into the prognostic value of individual mutations and combinations thereof is warranted.
Keywords: cholangiocarcinoma; genetic; genomic; mutation; radiotherapy.
Conflict of interest statement
B.D. reports consulting honoraria from Sermo. I.A.G. reports honoraria from AstraZeneca and support for meetings and/or travel from Roche. E.B.H. reports research funding from Merck Serono. C.T. reports a consulting/advisory role with Accuray. E.J.K. reports grants from National Institutes of Health, Stand Up 2 Cancer, MD Anderson Cancer Center, Philips Healthcare, Elekta, and GE Healthcare; personal fees from RenovoRx and Taylor and Francis; and a consulting/advisory role with Augmenix. A.C.K. reports ownership of shares in Aravive, Inc. P.D. reports consulting/advisory relationships with the American Society for Radiation Oncology and the National Cancer Institute. All reported conflicts are outside of the submitted work and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. All other authors report no potential conflicts.
Figures

References
-
- Sebastian N.T., Tan Y., Miller E.D., Williams T.M., Diaz D.A. Stereotactic body radiation therapy is associated with improved overall survival compared to chemoradiation or radioembolization in the treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Transl. Radiat. Oncol. 2019;19:66–71. doi: 10.1016/j.ctro.2019.07.007. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

