Multicenter Technical Validation of 30 Rapid Antigen Tests for the Detection of SARS-CoV-2 (VALIDATE)
- PMID: 34946190
- PMCID: PMC8704317
- DOI: 10.3390/microorganisms9122589
Multicenter Technical Validation of 30 Rapid Antigen Tests for the Detection of SARS-CoV-2 (VALIDATE)
Abstract
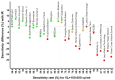
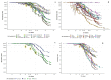
During COVID19 pandemic, SARS-CoV-2 rapid antigen tests (RATs) were marketed with minimal or no performance data. We aimed at closing this gap by determining technical sensitivities and specificities of 30 RATs prior to market release. We developed a standardized technical validation protocol and assessed 30 RATs across four diagnostic laboratories. RATs were tested in parallel using the Standard Q® (SD Biosensor/Roche) assay as internal reference. We used left-over universal transport/optimum media from nasopharyngeal swabs of 200 SARS-CoV-2 PCR-negative and 100 PCR-positive tested patients. Transport media was mixed with assay buffer and applied to RATs according to manufacturer instructions. Sensitivities were determined according to viral loads. Specificity of at least 99% and sensitivity of 95%, 90%, and 80% had to be reached for 107, 106, 105 virus copies/mL, respectively. Sensitivities ranged from 43.5% to 98.6%, 62.3% to 100%, and 66.7% to 100% at 105, 106, 107 copies/mL, respectively. Automated assay readers such as ExDia or LumiraDx showed higher performances. Specificities ranged from 88.8% to 100%. Only 15 of 30 (50%) RATs passed our technical validation. Due to the high failure rate of 50%, mainly caused by lack of sensitivity, we recommend a thorough validation of RATs prior to market release.
Keywords: COVID-19; SARS-CoV-2; diagnostics; rapid antigen test; virus testing.
Conflict of interest statement
G.G. is medical advisor of Resistell, a start-up developing nanomotion instruments to assess the antibiotic susceptibility of bacteria. G.G. also reports research grants from Resistell (Switzerland) and Nittobo (Japan), outside the submitted work. Moreover, G.G. is the co-director of “JeuPro”, a start-up distributing the game Krobs, a card game about microbes’ transmission. G.G. and A.C. reports grants from Becton Dickinson, outside the submitted work. All other authors declare no conflict of interest.
Figures

References
-
- WHO Rolling Updates on Coronavirus Disease (COVID-19) [(accessed on 25 January 2021)]; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-a....
-
- WHO Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays. [(accessed on 16 July 2021)]; Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnos....
-
- FIND SARS-CoV-2 Diagnostic Pipeline. [(accessed on 25 January 2021)]. Available online: https://www.finddx.org/covid-19/pipeline/?avance=all&type=Rapid+diagnost....
-
- Caruana G., Croxatto A., Kampouri E., Kritikos A., Opota O., Foerster M., Brouillet R., Senn L., Lienhard R., Egli A., et al. Implementing SARS-CoV-2 Rapid Antigen Testing in the Emergency Ward of a Swiss University Hospital: The INCREASE Study. Microorganisms. 2021;9:798. doi: 10.3390/microorganisms9040798. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

