Novel Therapies of Hepatitis B and D
- PMID: 34946209
- PMCID: PMC8707465
- DOI: 10.3390/microorganisms9122607
Novel Therapies of Hepatitis B and D
Abstract
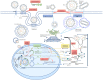
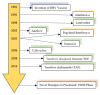
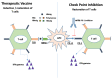
Hepatitis B virus (HBV) infection is a global public health issue and is a major cause of cirrhosis and hepatocellular carcinoma (HCC). Hepatitis D virus (HDV) requires the hepatitis B surface antigen (HBsAg) to replicate. The eradication of HBV, therefore, can also cure HDV. The current therapies for chronic hepatitis B and D are suboptimal and cannot definitely cure the viruses. In order to achieve functional or complete cure of these infections, novel therapeutic agents that target the various sites of the viral replicative cycle are necessary. Furthermore, novel immunomodulatory agents are also essential to achieve viral clearance. Many of these new promising compounds such as entry inhibitors, covalently closed circular DNA (cccDNA) inhibitors, small interfering RNAs (siRNAs), capsid assembly modulators and nucleic acid polymers are in various stages of clinical developments. In this review article, we provided a comprehensive overview of the structure and lifecycle of HBV, the limitations of the current therapies and a summary of the novel therapeutic agents for both HDV and HBV infection.
Keywords: Hepatitis B virus (HBV); Hepatitis D virus (HDV); NRTIs; capsid assembly modulators; cccDNA inhibitors; entry and exit inhibitors; immunomodulators; interferon; novel therapies; siRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization. Hepatitis B. World Health Organization. [(accessed on 1 October 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
-
- McMahon B.J. Seminars in Liver Disease. Volume 25. Thieme Medical Publishers, Inc.; New York, NY, USA: 2005. Epidemiology and natural history of hepatitis B; pp. 3–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

