Prediction of Blood-Brain Barrier Penetration (BBBP) Based on Molecular Descriptors of the Free-Form and In-Blood-Form Datasets
- PMID: 34946509
- PMCID: PMC8708321
- DOI: 10.3390/molecules26247428
Prediction of Blood-Brain Barrier Penetration (BBBP) Based on Molecular Descriptors of the Free-Form and In-Blood-Form Datasets
Abstract
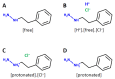
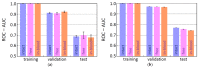
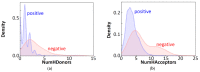
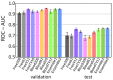
The blood-brain barrier (BBB) controls the entry of chemicals from the blood to the brain. Since brain drugs need to penetrate the BBB, rapid and reliable prediction of BBB penetration (BBBP) is helpful for drug development. In this study, free-form and in-blood-form datasets were prepared by modifying the original BBBP dataset, and the effects of the data modification were investigated. For each dataset, molecular descriptors were generated and used for BBBP prediction by machine learning (ML). For ML, the dataset was split into training, validation, and test data by the scaffold split algorithm MoleculeNet used. This creates an unbalanced split and makes the prediction difficult; however, we decided to use that algorithm to evaluate the predictive performance for unknown compounds dissimilar to existing ones. The highest prediction score was obtained by the random forest model using 212 descriptors from the free-form dataset, and this score was higher than the existing best score using the same split algorithm without using any external database. Furthermore, using a deep neural network, a comparable result was obtained with only 11 descriptors from the free-form dataset, and the resulting descriptors suggested the importance of recognizing the glucose-like characteristics in BBBP prediction.
Keywords: blood-brain barrier penetration (BBBP); deep neural network (DNN); forward search; free-form dataset; in-blood-form dataset; machine learning (ML); molecular descriptor; random forest (RF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





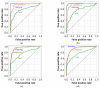
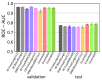
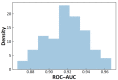
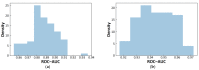
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

