Highly Efficient Kinetic Resolution of Aryl-Alkenyl Alcohols by Ru-Catalyzed Hydrogen Transfer
- PMID: 34946557
- PMCID: PMC8705739
- DOI: 10.3390/molecules26247475
Highly Efficient Kinetic Resolution of Aryl-Alkenyl Alcohols by Ru-Catalyzed Hydrogen Transfer
Abstract
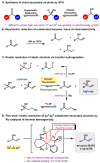
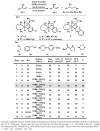
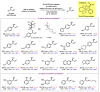
No matter through asymmetric reduction of ketones or kinetic resolution of secondary alcohols, enantioselective synthesis of the corresponding secondary alcohols is challenging when the two groups attached to the prochiral or chiral centers are spatially or electronically similar. For examples, dialkyl (sp3 vs. sp3), diaryl (sp2 vs. sp2), and aryl-alkenyl (sp2 vs. sp2) alcohols are difficult to produce with high enantioselectivities. By exploiting our recently developed Ru-catalysts of minimal stereogenicity, we reported herein a highly efficient kinetic resolution of aryl-alkenyl alcohols through hydrogen transfer. This method enabled such versatile chiral building blocks for organic synthesis as allylic alcohols, to be readily accessed with excellent enantiomeric excesses at practically useful conversions.
Keywords: Ru-catalyst; aryl-alkenyl alcohols; asymmetric transfer hydrogenation; kinetic resolution; selectivity factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Asymmetric Transfer Hydrogenation of Densely Functionalized Diheteroaryl and Diaryl Ketones by a Ru-Catalyst of Minimal Stereogenicity.Org Lett. 2020 Nov 6;22(21):8458-8463. doi: 10.1021/acs.orglett.0c03064. Epub 2020 Oct 12. Org Lett. 2020. PMID: 33044077
-
Iron-, Cobalt-, and Nickel-Catalyzed Asymmetric Transfer Hydrogenation and Asymmetric Hydrogenation of Ketones.Acc Chem Res. 2015 Sep 15;48(9):2587-98. doi: 10.1021/acs.accounts.5b00043. Epub 2015 Aug 24. Acc Chem Res. 2015. PMID: 26301426
-
Kinetic Resolution of Tertiary 2-Alkoxycarboxamido-Substituted Allylic Alcohols by Chiral Phosphoric Acid Catalyzed Intramolecular Transesterification.Angew Chem Int Ed Engl. 2019 Jul 22;58(30):10315-10319. doi: 10.1002/anie.201905034. Epub 2019 Jun 25. Angew Chem Int Ed Engl. 2019. PMID: 31134717
-
Ketoreductase Catalyzed (Dynamic) Kinetic Resolution for Biomanufacturing of Chiral Chemicals.Front Bioeng Biotechnol. 2022 Jun 30;10:929784. doi: 10.3389/fbioe.2022.929784. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35845398 Free PMC article. Review.
-
Benzothiazoline: versatile hydrogen donor for organocatalytic transfer hydrogenation.Acc Chem Res. 2015 Feb 17;48(2):388-98. doi: 10.1021/ar500414x. Epub 2015 Jan 22. Acc Chem Res. 2015. PMID: 25611073 Review.
References
-
- Noyori R., Hashiguchi S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Acc. Chem. Res. 1997;30:97–102. doi: 10.1021/ar9502341. - DOI
-
- Palmer M.J., Wills M. Asymmetric transfer hydrogenation of C=O and C=N bonds. Tetrahedron Asymmetry. 1999;10:2045–2061. doi: 10.1016/S0957-4166(99)00216-5. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

