Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway
- PMID: 34946711
- PMCID: PMC8708443
- DOI: 10.3390/molecules26247629
Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway
Abstract
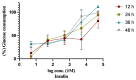
Insulin resistance contributes to several disorders including type 2 diabetes and cardiovascular diseases. Carpachromene is a natural active compound that inhibits α-glucosidase enzyme. The aim of the present study is to investigate the potential activity of carpachromene on glucose consumption, metabolism and insulin signalling in a HepG2 cells insulin resistant model. A HepG2 insulin resistant cell model (HepG2/IRM) was established. Cell viability assay of HepG2/IRM cells was performed after carpachromene/metformin treatment. Glucose concentration and glycogen content were determined. Western blot analysis of insulin receptor, IRS1, IRS2, PI3k, Akt, GSK3, FoxO1 proteins after carpachromene treatment was performed. Phosphoenolpyruvate carboxykinase (PEPCK) and hexokinase (HK) enzymes activity was also estimated. Viability of HepG2/IRM cells was over 90% after carpachromene treatment at concentrations 6.3, 10, and 20 µg/mL. Treatment of HepG2/IRM cells with carpachromene decreased glucose concentration in a concentration- and time-dependant manner. In addition, carpachromene increased glycogen content of HepG2/IRM cells. Moreover, carpachromene treatment of HepG2/IRM cells significantly increased the expression of phosphorylated/total ratios of IR, IRS1, PI3K, Akt, GSK3, and FoxO1 proteins. Furthermore, PEPCK enzyme activity was significantly decreased, and HK enzyme activity was significantly increased after carpachromene treatment. The present study examined, for the first time, the potential antidiabetic activity of carpachromene on a biochemical and molecular basis. It increased the expression ratio of insulin receptor and IRS1 which further phosphorylated/activated PI3K/Akt pathway and phosphorylated/inhibited GSK3 and FoxO1 proteins. Our findings revealed that carpachromene showed central molecular regulation of glucose metabolism and insulin signalling via IR/IRS1/ PI3K/Akt/GSK3/FoxO1 pathway.
Keywords: HepG2 cells; PI3K/Akt/GSK3/FoxO1 pathway; carpachromene; insulin receptor; insulin resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Abdel-Hamid N., Fathy M., Amgad S.W. Glycoregulatory Enzymes as Early Diagnostic Markers during Premalignant Stage in Hepatocellular Carcinoma. Am. J. Cancer Prev. 2013;1:14–19. doi: 10.12691/ajcp-1-2-1. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

