Naturally Occurring Chromone Glycosides: Sources, Bioactivities, and Spectroscopic Features
- PMID: 34946728
- PMCID: PMC8704703
- DOI: 10.3390/molecules26247646
Naturally Occurring Chromone Glycosides: Sources, Bioactivities, and Spectroscopic Features
Abstract
Chromone glycosides comprise an important group of secondary metabolites. They are widely distributed in plants and, to a lesser extent, in fungi and bacteria. Significant biological activities, including antiviral, anti-inflammatory, antitumor, antimicrobial, etc., have been discovered for chromone glycosides, suggesting their potential as drug leads. This review compiles 192 naturally occurring chromone glycosides along with their sources, classification, biological activities, and spectroscopic features. Detailed biosynthetic pathways and chemotaxonomic studies are also described. Extensive spectroscopic features for this class of compounds have been thoroughly discussed, and detailed 13C-NMR data of compounds 1-192, have been added, except for those that have no reported 13C-NMR data.
Keywords: 13C-NMR data; activity; benzo-γ-pyrone; chemical structure; chromone glycosides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
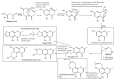

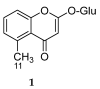
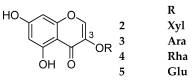
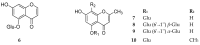
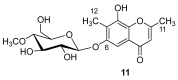
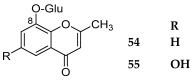

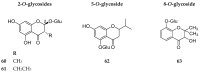





References
-
- Machado N.F.L., Marques M.P.M. Bioactive Chromone Derivatives-Structural Diversity. Curr. Bioact. Compd. 2010;6:76–89. doi: 10.2174/157340710791184859. - DOI
-
- Daniel M. Medicinal Plants: Chemistry and Properties. Science Publishers; Hauppauge, NY, USA: 2006.
-
- Mander L., Liu H.-W. Comprehensive Natural Products II: Chemistry and Biology. Volume 1. Elsevier; Amsterdam, The Netherlands: 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

