Genome-Wide Analyses for Osteosarcoma in Leonberger Dogs Reveal the CDKN2A/B Gene Locus as a Major Risk Locus
- PMID: 34946912
- PMCID: PMC8700858
- DOI: 10.3390/genes12121964
Genome-Wide Analyses for Osteosarcoma in Leonberger Dogs Reveal the CDKN2A/B Gene Locus as a Major Risk Locus
Abstract
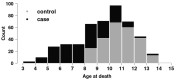
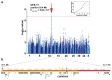
Dogs represent a unique spontaneous cancer model. Osteosarcoma (OSA) is the most common primary bone tumor in dogs (OMIA 001441-9615), and strongly resembles human forms of OSA. Several large- to giant-sized dog breeds, including the Leonberger, have a greatly increased risk of developing OSA. We performed genome-wide association analysis with high-density imputed SNP genotype data from 273 Leonberger cases with a median age of 8.1 [3.1-13.5] years and 365 controls older than eight years. This analysis revealed significant associations at the CDKN2A/B gene locus on canine chromosome 11, mirroring previous findings in other dog breeds, such as the greyhound, that also show an elevated risk for OSA. Heritability (h2SNP) was determined to be 20.6% (SE = 0.08; p-value = 5.7 × 10-4) based on a breed prevalence of 20%. The 2563 SNPs across the genome accounted for nearly all the h2SNP of OSA, with 2183 SNPs of small effect, 316 SNPs of moderate effect, and 64 SNPs of large effect. As with many other cancers it is likely that regulatory, non-coding variants underlie the increased risk for cancer development. Our findings confirm a complex genetic basis of OSA, moderate heritability, and the crucial role of the CDKN2A/B locus leading to strong cancer predisposition in dogs. It will ultimately be interesting to study and compare the known genetic loci associated with canine OSA in human OSA.
Keywords: CDKN2A/B; Leonberger; animal model; bone cancer; canis familiaris; osteosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Genome-wide analyses implicate 33 loci in heritable dog osteosarcoma, including regulatory variants near CDKN2A/B.Genome Biol. 2013 Dec 12;14(12):R132. doi: 10.1186/gb-2013-14-12-r132. Genome Biol. 2013. PMID: 24330828 Free PMC article.
-
Functional Genetic Single-Nucleotide Polymorphisms (SNPs) in Cyclin-Dependent Kinase Inhibitor 2A/B (CDKN2A/B) Locus Are Associated with Risk and Prognosis of Osteosarcoma in Chinese Populations.Med Sci Monit. 2019 Feb 18;25:1307-1313. doi: 10.12659/MSM.915001. Med Sci Monit. 2019. PMID: 30774116 Free PMC article.
-
Identification of common predisposing loci to hematopoietic cancers in four dog breeds.PLoS Genet. 2021 Apr 1;17(4):e1009395. doi: 10.1371/journal.pgen.1009395. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33793571 Free PMC article.
-
Functional genomics of the CDKN2A/B locus in cardiovascular and metabolic disease: what have we learned from GWASs?Trends Endocrinol Metab. 2015 Apr;26(4):176-84. doi: 10.1016/j.tem.2015.01.008. Epub 2015 Mar 3. Trends Endocrinol Metab. 2015. PMID: 25744911 Review.
-
Comparative biology of human and canine osteosarcoma.Anticancer Res. 2007 Jan-Feb;27(1A):155-64. Anticancer Res. 2007. PMID: 17352227 Review.
Cited by
-
Genome-wide association studies of human and rat BMI converge on synapse, epigenome, and hormone signaling networks.Cell Rep. 2023 Aug 29;42(8):112873. doi: 10.1016/j.celrep.2023.112873. Epub 2023 Jul 31. Cell Rep. 2023. PMID: 37527041 Free PMC article.
-
Sarcoma Predisposition in Dogs with a Comparative View to Human Orthologous Disease.Vet Sci. 2023 Jul 21;10(7):476. doi: 10.3390/vetsci10070476. Vet Sci. 2023. PMID: 37505880 Free PMC article. Review.
-
Clinical Use of Molecular Biomarkers in Canine and Feline Oncology: Current and Future.Vet Sci. 2024 May 2;11(5):199. doi: 10.3390/vetsci11050199. Vet Sci. 2024. PMID: 38787171 Free PMC article. Review.
-
Dog breeds and conformations predisposed to osteosarcoma in the UK: a VetCompass study.Canine Med Genet. 2023 Jun 27;10(1):8. doi: 10.1186/s40575-023-00131-2. Canine Med Genet. 2023. PMID: 37365662 Free PMC article.
-
Establishment and Characterization of Cell Lines from Canine Metastatic Osteosarcoma.Cells. 2023 Dec 21;13(1):25. doi: 10.3390/cells13010025. Cells. 2023. PMID: 38201229 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

