Fungal Laccases: The Forefront of Enzymes for Sustainability
- PMID: 34947030
- PMCID: PMC8708107
- DOI: 10.3390/jof7121048
Fungal Laccases: The Forefront of Enzymes for Sustainability
Abstract
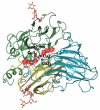
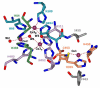
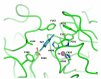
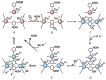
Enzymatic catalysis is one of the main pillars of sustainability for industrial production. Enzyme application allows minimization of the use of toxic solvents and to valorize the agro-industrial residues through reuse. In addition, they are safe and energy efficient. Nonetheless, their use in biotechnological processes is still hindered by the cost, stability, and low rate of recycling and reuse. Among the many industrial enzymes, fungal laccases (LCs) are perfect candidates to serve as a biotechnological tool as they are outstanding, versatile catalytic oxidants, only requiring molecular oxygen to function. LCs are able to degrade phenolic components of lignin, allowing them to efficiently reuse the lignocellulosic biomass for the production of enzymes, bioactive compounds, or clean energy, while minimizing the use of chemicals. Therefore, this review aims to give an overview of fungal LC, a promising green and sustainable enzyme, its mechanism of action, advantages, disadvantages, and solutions for its use as a tool to reduce the environmental and economic impact of industrial processes with a particular insight on the reuse of agro-wastes.
Keywords: agro-wastes; catalysis; enzymes; fungal laccase; immobilization; solid state fermentation; sustainability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chapman J., Ismail A.E., Dinu C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts. 2018;8:238. doi: 10.3390/catal8060238. - DOI
-
- European Commission Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Next Steps for a Sustainable European Future European Action for Sustainability. 2016. [(accessed on 25 February 2021)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52016DC073....
-
- Grand View Research. [(accessed on 8 April 2021)]. Available online: https://www.grandviewresearch.com/industry-analysis/enzymes-industry.
-
- Global Market Insight. [(accessed on 8 April 2021)]. Available online: https://www.gminsights.com/industry-analysis/enzymes-market.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

