A Visual and Comprehensive Review on COVID-19-Associated Pulmonary Aspergillosis (CAPA)
- PMID: 34947049
- PMCID: PMC8708864
- DOI: 10.3390/jof7121067
A Visual and Comprehensive Review on COVID-19-Associated Pulmonary Aspergillosis (CAPA)
Abstract
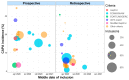
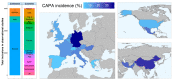
Coronavirus disease 19 (COVID-19)-associated pulmonary aspergillosis (CAPA) is a severe fungal infection complicating critically ill COVID-19 patients. Numerous retrospective and prospective studies have been performed to get a better grasp on this lethal co-infection. We performed a qualitative review and summarized data from 48 studies in which 7047 patients had been included, of whom 820 had CAPA. The pooled incidence of proven, probable or putative CAPA was 15.1% among 2953 ICU-admitted COVID-19 patients included in 18 prospective studies. Incidences showed great variability due to multiple factors such as discrepancies in the rate and depth of the fungal work-up. The pathophysiology and risk factors for CAPA are ill-defined, but therapy with corticosteroids and anti-interleukin-6 therapy potentially confer the biggest risk. Sampling for mycological work-up using bronchoscopy is the cornerstone for diagnosis, as imaging is often aspecific. CAPA is associated with an increased mortality, but we do not have conclusive data whether therapy contributes to an increased survival in these patients. We conclude our review with a comparison between influenza-associated pulmonary aspergillosis (IAPA) and CAPA.
Keywords: COVID-19; COVID-19-associated pulmonary aspergillosis (CAPA); IAPA; aspergillosis; critical care; influenza; influenza-associated pulmonary aspergillosis; intensive care unit.
Conflict of interest statement
S.F. is funded by a Research Foundation Flanders (FWO) PhD fellowship (11M6922N) and declares travel support from Pfizer. K.L. received consultancy fees from MRM Health, MSD and Gilead, speaker fees from FUJIFILM WAKO, Pfizer and Gilead and a service fee from Thermo Fisher Scientific. I.S. is supported by the Clinical Research Fund of UZ Leuven and served as a consultant to and has received unrestricted travel and research grants from Gilead Sciences, Merck Sharpe and Dohme Corp., Pfizer, Inc. and Cidara. G.D. declares advisory board participation (Pfizer, Gilead) and lecture fees from Gilead and Pfizer. J.W. has received investigator-initiated grants from Pfizer, Gilead and MSD and speakers’ and travel fees from Pfizer, Gilead and MSD. The other authors report no conflict of interest.
Figures


References
-
- Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., Lagrou K., Verweij P.E., Van de Veerdonk F.L., Gommers D., et al. Invasive Aspergillosis in Patients Admitted to the Intensive Care Unit with Severe Influenza: A Retrospective Cohort Study. Lancet Respir. Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. - DOI - PubMed
-
- Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., Clancy C.J., Wingard J.R., Lockhart S.R., Groll A.H., et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. - DOI - PMC - PubMed
-
- Blot S.I., Taccone F.S., Van Den Abeele A.M., Bulpa P., Meersseman W., Brusselaers N., Dimopoulos G., Paiva J.A., Misset B., Rello J., et al. A Clinical Algorithm to Diagnose Invasive Pulmonary Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

