Biocompatibility and Degradation Behavior of Molybdenum in an In Vivo Rat Model
- PMID: 34947370
- PMCID: PMC8705131
- DOI: 10.3390/ma14247776
Biocompatibility and Degradation Behavior of Molybdenum in an In Vivo Rat Model
Abstract
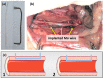
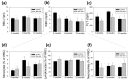
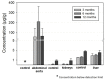
The biocompatibility and degradation behavior of pure molybdenum (Mo) as a bioresorbable metallic material (BMM) for implant applications were investigated. In vitro degradation of a commercially available Mo wire (ø250 µm) was examined after immersion in modified Kokubo's SBF for 28 days at 37 °C and pH 7.4. For assessment of in vivo degradation, the Mo wire was implanted into the abdominal aorta of female Wistar rats for 3, 6 and 12 months. Microstructure and corrosion behavior were analyzed by means of SEM/EDX analysis. After explantation, Mo levels in serum, urine, aortic vessel wall and organs were investigated via ICP-OES analysis. Furthermore, histological analyses of the liver, kidneys, spleen, brain and lungs were performed, as well as blood count and differentiation by FACS analysis. Levels of the C-reactive protein were measured in blood plasma of all the animals. In vitro and in vivo degradation behavior was very similar, with formation of uniform, non-passivating and dissolving product layers without occurrence of a localized corrosion attack. The in vitro degradation rate was 101.6 µg/(cm2·d) which corresponds to 33.6 µm/y after 28 days. The in vivo degradation rates of 12, 33 and 36 µg/(cm2·d) were observed after 3, 6 and 12 months for the samples properly implanted in the aortic vessel wall. This corresponds with a degradation rate of 13.5 µm/y for the 12-month cohort. However, the magnitude of degradation strongly depended on the implant site, with the wires incorporated into the vessel wall showing the most severe degradation. Degradation of the implanted Mo wire neither induced an increase in serum or urine Mo levels nor were elevated Mo levels found in the liver and kidneys compared with the respective controls. Only in the direct vicinity of the implant in the aortic vessel wall, a significant amount of Mo was found, which, however, was far below the amounts to be expected from degrading wires. No abnormalities were detected for all timepoints in histological and blood analyses compared to the control group. The C-reactive protein levels were similar between all the groups, indicating no inflammation processes. These findings suggest that dissolved Mo from a degrading implant is physiologically transported and excreted. Furthermore, radiographic and µCT analyses revealed excellent radiopacity of Mo in tissues. These findings and the unique combination with its extraordinary mechanical properties make Mo an interesting alternative for established BMMs.
Keywords: biocompatibility; bioresorbable; blood analysis; degradation; histologic analysis; in vivo; molybdenum; organ accumulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mater. Sci. Eng. R. 2014;77:1–34. doi: 10.1016/j.mser.2014.01.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

