Impaired Retromer Function in Niemann-Pick Type C Disease Is Dependent on Intracellular Cholesterol Accumulation
- PMID: 34948052
- PMCID: PMC8705785
- DOI: 10.3390/ijms222413256
Impaired Retromer Function in Niemann-Pick Type C Disease Is Dependent on Intracellular Cholesterol Accumulation
Abstract
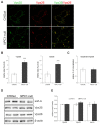
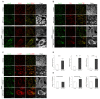
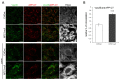
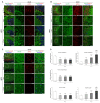
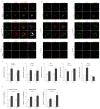
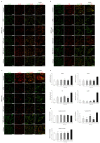
Niemann-Pick type C disease (NPC) is a rare inherited neurodegenerative disorder characterized by an accumulation of intracellular cholesterol within late endosomes and lysosomes due to NPC1 or NPC2 dysfunction. In this work, we tested the hypothesis that retromer impairment may be involved in the pathogenesis of NPC and may contribute to increased amyloidogenic processing of APP and enhanced BACE1-mediated proteolysis observed in NPC disease. Using NPC1-null cells, primary mouse NPC1-deficient neurons and NPC1-deficient mice (BALB/cNctr-Npc1m1N), we show that retromer function is impaired in NPC. This is manifested by altered transport of the retromer core components Vps26, Vps35 and/or retromer receptor sorLA and by retromer accumulation in neuronal processes, such as within axonal swellings. Changes in retromer distribution in NPC1 mouse brains were observed already at the presymptomatic stage (at 4-weeks of age), indicating that the retromer defect occurs early in the course of NPC disease and may contribute to downstream pathological processes. Furthermore, we show that cholesterol depletion in NPC1-null cells and in NPC1 mouse brains reverts retromer dysfunction, suggesting that retromer impairment in NPC is mechanistically dependent on cholesterol accumulation. Thus, we characterized retromer dysfunction in NPC and propose that the rescue of retromer impairment may represent a novel therapeutic approach against NPC.
Keywords: NPC1; cholesterol homeostasis; endolysosomal pathway; neurodegeneration; neurodegenerative diseases; rare diseases; retromer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Beneficial effects of primidone in Niemann-Pick disease type C (NPC)-model cells and mice: Reduction of unesterified cholesterol levels in cells and extension of lifespan in mice.Eur J Pharmacol. 2021 Apr 5;896:173907. doi: 10.1016/j.ejphar.2021.173907. Epub 2021 Jan 24. Eur J Pharmacol. 2021. PMID: 33503462
-
Targeting defective sphingosine kinase 1 in Niemann-Pick type C disease with an activator mitigates cholesterol accumulation.J Biol Chem. 2020 Jul 3;295(27):9121-9133. doi: 10.1074/jbc.RA120.012659. Epub 2020 May 8. J Biol Chem. 2020. PMID: 32385114 Free PMC article.
-
Cholesterol accumulation in Niemann Pick type C (NPC) model cells causes a shift in APP localization to lipid rafts.Biochem Biophys Res Commun. 2010 Mar 12;393(3):404-9. doi: 10.1016/j.bbrc.2010.02.007. Epub 2010 Feb 6. Biochem Biophys Res Commun. 2010. PMID: 20138836 Free PMC article.
-
Laboratory diagnosis of the Niemann-Pick type C disease: an inherited neurodegenerative disorder of cholesterol metabolism.Metab Brain Dis. 2019 Oct;34(5):1253-1260. doi: 10.1007/s11011-019-00445-w. Epub 2019 Jun 13. Metab Brain Dis. 2019. PMID: 31197681 Free PMC article. Review.
-
Niemann-Pick Disease Type C: from molecule to clinic.Clin Exp Pharmacol Physiol. 2010 Jan;37(1):132-40. doi: 10.1111/j.1440-1681.2009.05235.x. Epub 2009 Jun 29. Clin Exp Pharmacol Physiol. 2010. PMID: 19566836 Review.
Cited by
-
Endo-lysosomal dysfunction and neuronal-glial crosstalk in Niemann-Pick type C disease.Philos Trans R Soc Lond B Biol Sci. 2024 Apr 8;379(1899):20220388. doi: 10.1098/rstb.2022.0388. Epub 2024 Feb 19. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38368932 Free PMC article. Review.
-
The ancestral type of the R-RAS protein has oncogenic potential.Cell Mol Biol Lett. 2024 Feb 21;29(1):27. doi: 10.1186/s11658-024-00546-0. Cell Mol Biol Lett. 2024. PMID: 38383288 Free PMC article.
-
Structure and function of cancer-related developmentally regulated GTP-binding protein 1 (DRG1) is conserved between sponges and humans.Sci Rep. 2022 Jul 5;12(1):11379. doi: 10.1038/s41598-022-15242-2. Sci Rep. 2022. PMID: 35790840 Free PMC article.
-
CD63 sorts cholesterol into endosomes for storage and distribution via exosomes.Nat Cell Biol. 2024 Jul;26(7):1093-1109. doi: 10.1038/s41556-024-01432-9. Epub 2024 Jun 17. Nat Cell Biol. 2024. PMID: 38886558
-
Adenosine A2A Receptor Activation Regulates Niemann-Pick C1 Expression and Localization in Macrophages.Curr Issues Mol Biol. 2023 Jun 7;45(6):4948-4969. doi: 10.3390/cimb45060315. Curr Issues Mol Biol. 2023. PMID: 37367064 Free PMC article.
References
-
- Cataldo A.M., Barnett J.L., Pieroni C., Nixon R.A. Increased Neuronal Endocytosis and Protease Delivery to Early Endosomes in Sporadic Alzheimer’s Disease: Neuropathologic Evidence for a Mechanism of Increased β-Amyloidogenesis. J. Neurosci. 1997;17:6142–6151. doi: 10.1523/JNEUROSCI.17-16-06142.1997. - DOI - PMC - PubMed
-
- Cataldo A.M., Petanceska S., Terio N.B., Peterhoff C.M., Durham R., Mercken M., Mehta P.D., Buxbaum J., Haroutunian V., Nixon R.A. Aβ localization in abnormal endosomes: Association with earliest Aβ elevations in AD and Down syndrome. Neurobiol. Aging. 2004;25:1263–1272. doi: 10.1016/j.neurobiolaging.2004.02.027. - DOI - PubMed
-
- Lauritzen I., Pardossi-Piquard R., Bourgeois A., Pagnotta S., Biferi M.G., Barkats M., Lacor P., Klein W., Bauer C., Checler F. Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology. Acta Neuropathol. 2016;132:257–276. doi: 10.1007/s00401-016-1577-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

