In Vitro Comparative Study of Solid Lipid and PLGA Nanoparticles Designed to Facilitate Nose-to-Brain Delivery of Insulin
- PMID: 34948054
- PMCID: PMC8703723
- DOI: 10.3390/ijms222413258
In Vitro Comparative Study of Solid Lipid and PLGA Nanoparticles Designed to Facilitate Nose-to-Brain Delivery of Insulin
Abstract
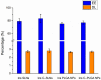
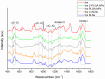
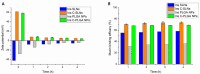
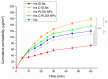
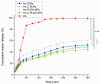
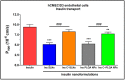
The brain insulin metabolism alteration has been addressed as a pathophysiological factor underlying Alzheimer's disease (AD). Insulin can be beneficial in AD, but its macro-polypeptide nature negatively influences the chances of reaching the brain. The intranasal (IN) administration of therapeutics in AD suggests improved brain-targeting. Solid lipid nanoparticles (SLNs) and poly(lactic-co-glycolic acid) nanoparticles (PLGA NPs) are promising carriers to deliver the IN-administered insulin to the brain due to the enhancement of the drug permeability, which can even be improved by chitosan-coating. In the present study, uncoated and chitosan-coated insulin-loaded SLNs and PLGA NPs were formulated and characterized. The obtained NPs showed desirable physicochemical properties supporting IN applicability. The in vitro investigations revealed increased mucoadhesion, nasal diffusion, and drug release rate of both insulin-loaded nanocarriers over native insulin with the superiority of chitosan-coated SLNs. Cell-line studies on human nasal epithelial and brain endothelial cells proved the safety IN applicability of nanoparticles. Insulin-loaded nanoparticles showed improved insulin permeability through the nasal mucosa, which was promoted by chitosan-coating. However, native insulin exceeded the blood-brain barrier (BBB) permeation compared with nanoparticulate formulations. Encapsulating insulin into chitosan-coated NPs can be beneficial for ensuring structural stability, enhancing nasal absorption, followed by sustained drug release.
Keywords: PLGA nanoparticles; blood-brain barrier permeability; chitosan-coating; insulin; mucoadhesion; nasal mucosa permeability; nose-to-brain delivery; solid lipid nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Heron M. Deaths: Leading Causes for 2016. Natl. Vital. Stat. Rep. 2018;67:1–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

