Live-Cell Imaging of Single Neurotrophin Receptor Molecules on Human Neurons in Alzheimer's Disease
- PMID: 34948057
- PMCID: PMC8708879
- DOI: 10.3390/ijms222413260
Live-Cell Imaging of Single Neurotrophin Receptor Molecules on Human Neurons in Alzheimer's Disease
Abstract
Neurotrophin receptors such as the tropomyosin receptor kinase A receptor (TrkA) and the low-affinity binding p75 neurotrophin receptor p75NTR play a critical role in neuronal survival and their functions are altered in Alzheimer's disease (AD). Changes in the dynamics of receptors on the plasma membrane are essential to receptor function. However, whether receptor dynamics are affected in different pathophysiological conditions is unexplored. Using live-cell single-molecule imaging, we examined the surface trafficking of TrkA and p75NTR molecules on live neurons that were derived from human-induced pluripotent stem cells (hiPSCs) of presenilin 1 (PSEN1) mutant familial AD (fAD) patients and non-demented control subjects. Our results show that the surface movement of TrkA and p75NTR and the activation of TrkA- and p75NTR-related phosphoinositide-3-kinase (PI3K)/serine/threonine-protein kinase (AKT) signaling pathways are altered in neurons that are derived from patients suffering from fAD compared to controls. These results provide evidence for altered surface movement of receptors in AD and highlight the importance of investigating receptor dynamics in disease conditions. Uncovering these mechanisms might enable novel therapies for AD.
Keywords: Alzheimer’s disease; TrkA; human-induced pluripotent stem cell; live-cell single-molecule imaging; neuronal; p75NTR; receptor dynamics.
Conflict of interest statement
J.K. and A.T. were the employee of BioTalentum Ltd. at the time when the published experiments were performed. A.D. is the director and owner of BioTalentum Ltd.
Figures

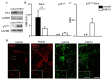
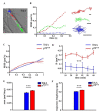
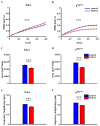


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

