Nano-Sized Extracellular Matrix Particles Lead to Therapeutic Improvement for Cutaneous Wound and Hindlimb Ischemia
- PMID: 34948061
- PMCID: PMC8705579
- DOI: 10.3390/ijms222413265
Nano-Sized Extracellular Matrix Particles Lead to Therapeutic Improvement for Cutaneous Wound and Hindlimb Ischemia
Abstract
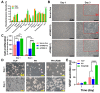
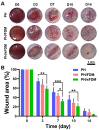
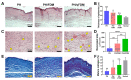
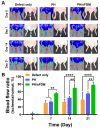
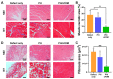
Cell-derived matrix (CDM) has proven its therapeutic potential and been utilized as a promising resource in tissue regeneration. In this study, we prepared a human fibroblast-derived matrix (FDM) by decellularization of in vitro cultured cells and transformed the FDM into a nano-sized suspended formulation (sFDM) using ultrasonication. The sFDM was then homogeneously mixed with Pluronic F127 and hyaluronic acid (HA), to effectively administer sFDM into target sites. Both sFDM and sFDM containing hydrogel (PH/sFDM) were characterized via immunofluorescence, sol-gel transition, rheological analysis, and biochemical factors array. We found that PH/sFDM hydrogel has biocompatible, mechanically stable, injectable properties and can be easily administered into the external and internal target regions. sFDM itself holds diverse bioactive molecules. Interestingly, sFDM-containing serum-free media helped maintain the metabolic activity of endothelial cells significantly better than those in serum-free condition. PH/sFDM also promoted vascular endothelial growth factor (VEGF) secretion from monocytes in vitro. Moreover, when we evaluated therapeutic effects of PH/sFDM via the murine full-thickness skin wound model, regenerative potential of PH/sFDM was supported by epidermal thickness, significantly more neovessel formation, and enhanced mature collagen deposition. The hindlimb ischemia model also found some therapeutic improvements, as assessed by accelerated blood reperfusion and substantially diminished necrosis and fibrosis in the gastrocnemius and tibialis muscles. Together, based on sFDM holding a strong therapeutic potential, our engineered hydrogel (PH/sFDM) should be a promising candidate in tissue engineering and regenerative medicine.
Keywords: Pluronic F127; decellularization; fibroblast-derived matrix (FDM); hindlimb ischemia; hyaluronic acid; suspended FDM; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jang W.B., Ji S.T., Park J.H., Kim Y.-J., Kang S., Lee N.-K., Kim J.S., Lim H.J., Choi J., Van Le T.H. Engineered M13 peptide carrier promotes angiogenic potential of patient-derived human cardiac progenitor cells and in vivo engraftment. Tissue Eng. Regen. Med. 2020;17:323–333. doi: 10.1007/s13770-020-00244-w. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

