Zinc Exposure Promotes Commensal-to-Pathogen Transition in Pseudomonas aeruginosa Leading to Mucosal Inflammation and Illness in Mice
- PMID: 34948118
- PMCID: PMC8705841
- DOI: 10.3390/ijms222413321
Zinc Exposure Promotes Commensal-to-Pathogen Transition in Pseudomonas aeruginosa Leading to Mucosal Inflammation and Illness in Mice
Abstract
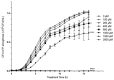
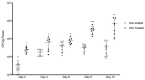
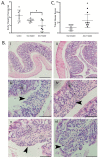
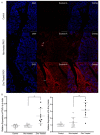
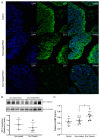
The opportunistic pathogen Pseudomonas aeruginosa (P. aeruginosa) is associated gastrointestinal (GI) inflammation and illness; however, factors motivating commensal-to-pathogen transition are unclear. Excessive zinc intake from supplements is common in humans. Due to the fact that zinc exposure enhances P. aeruginosa colonization in vitro, we hypothesized zinc exposure broadly activates virulence mechanisms, leading to inflammation and illness. P. aeruginosa was treated with excess zinc and growth, expression and secretion of key virulence factors, and biofilm production were determined. Effects on invasion, barrier function, and cytotoxicity were evaluated in Caco-2 cells co-cultured with P. aeruginosa pre-treated with zinc. Effects on colonization, mucosal pathology, inflammation, and illness were evaluated in mice infected with P. aeruginosa pre-treated with zinc. We found the expression and secretion of key virulence factors involved in quorum sensing (QS), motility (type IV pili, flagella), biosurfactants (rhamnolipids), toxins (exotoxin A), zinc homeostasis (CzcR), and biofilm production, were all significantly increased. Zinc exposure significantly increased P. aeruginosa invasion, permeability and cytotoxicity in Caco-2 cells, and enhanced colonization, inflammation, mucosal damage, and illness in mice. Excess zinc exposure has broad effects on key virulence mechanisms promoting commensal-to-pathogen transition of P. aeruginosa and illness in mice, suggesting excess zinc intake may have adverse effects on GI health in humans.
Keywords: Caco-2 cells; Pseudomonas aeruginosa; colonic inflammation; intestinal permeability; virulence; zinc.
Conflict of interest statement
All authors declare they have no competing interest.
Figures


References
-
- Kerckhoffs A.P., Ben-Amor K., Samsom M., van der Rest M.E., de Vogel J., Knol J., Akkermans L.M. Molecular analysis of faecal and duodenal samples reveals significantly higher prevalence and numbers of Pseudomonas aeruginosa in irritable bowel syndrome. J. Med. Microbiol. 2011;60:236–245. doi: 10.1099/jmm.0.022848-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

