Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations
- PMID: 34948167
- PMCID: PMC8705916
- DOI: 10.3390/ijms222413371
Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations
Abstract
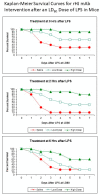
To determine whether mitigating the harmful effects of circulating microvesicle-associated inducible nitric oxide (MV-A iNOS) in vivo increases the survival of challenged mice in three different mouse models of sepsis, the ability of anti-MV-A iNOS monoclonal antibodies (mAbs) to rescue challenged mice was assessed using three different mouse models of sepsis. The vivarium of a research laboratory Balb/c mice were challenged with an LD80 dose of either lipopolysaccharide (LPS/endotoxin), TNFα, or MV-A iNOS and then treated at various times after the challenge with saline as control or with an anti-MV-A iNOS mAb as a potential immunotherapeutic to treat sepsis. Each group of mice was checked daily for survivors, and Kaplan-Meier survival curves were constructed. Five different murine anti-MV-A iNOS mAbs from our panel of 24 murine anti-MV-A iNOS mAbs were found to rescue some of the challenged mice. All five murine mAbs were used to genetically engineer humanized anti-MV-A iNOS mAbs by inserting the murine complementarity-determining regions (CDRs) into a human IgG1,kappa scaffold and expressing the humanized mAbs in CHO cells. Three humanized anti-MV-A iNOS mAbs were effective at rescuing mice from sepsis in three different animal models of sepsis. The effectiveness of the treatment was both time- and dose-dependent. Humanized anti-MV-A iNOS rHJ mAb could rescue up to 80% of the challenged animals if administered early and at a high dose. Our conclusions are that MV-A iNOS is a novel therapeutic target to treat sepsis; anti-MV-A iNOS mAbs can mitigate the harmful effects of MV-A iNOS; the neutralizing mAb's efficacy is both time- and dose-dependent; and a specifically targeted immunotherapeutic for MV-A iNOS could potentially save tens of thousands of lives annually and could result in improved antibiotic stewardship.
Keywords: iNOS; inducible nitric oxide synthase; microvesicles; nanoparticles; sepsis; therapeutic target.
Conflict of interest statement
R.J.W. and D.S.W. are both paid executives and partial owners of Research & Diagnostic Antibodies, the sponsor of the studies reported in this article. R.M.S. reports no conflicts of interest.
Figures



References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

