Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas
- PMID: 34948183
- PMCID: PMC8703592
- DOI: 10.3390/ijms222413388
Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas
Abstract
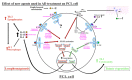
The impact of new and emerging therapies on the microenvironment of primary cutaneous lymphomas (PCLs) has been recently raised in the literature. Concomitantly, novel treatments are already used or registered (dupilumab, upadacitinib) and others seem to be added to the armamentarium against atopic dermatitis. Our aim was to review the literature on interleukins 4, 13, 22, and 31, and JAK/STAT pathways in PCLs to elucidate the safety of using biologics (dupilumab, tralokinumab, fezakinumab, nemolizumab) and small molecule inhibitors (upadacitinib, baricitinib, abrocitinib, ruxolitinib, tofacitinib) in the treatment of atopic dermatitis. We summarized the current state of knowledge on this topic based on the search of the PubMed database and related references published before 21 October 2021. Our analysis suggests that some of the mentioned agents (dupilumab, ruxolitinib) and others may have a direct impact on the progression of cutaneous lymphomas. This issue requires further study and meticulous monitoring of patients receiving these drugs to ensure their safety, especially in light of the FDA warning on tofacitinib. In conclusion, in the case of the rapid progression of atopic dermatitis/eczema, especially in patients older than 40 years old, there is a necessity to perform a biopsy followed by a very careful pathological examination.
Keywords: JAK-STAT pathway; Sézary syndrome; atopic dermatitis; biologic treatment; cutaneous lymphoma; cytokine; interleukins; mycosis fungoides; small molecule inhibitors; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

