A Complex Evaluation of the In-Vivo Biocompatibility and Degradation of an Extruded ZnMgSr Absorbable Alloy Implanted into Rabbit Bones for 360 Days
- PMID: 34948238
- PMCID: PMC8706155
- DOI: 10.3390/ijms222413444
A Complex Evaluation of the In-Vivo Biocompatibility and Degradation of an Extruded ZnMgSr Absorbable Alloy Implanted into Rabbit Bones for 360 Days
Abstract
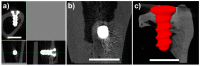
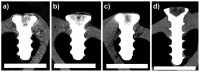
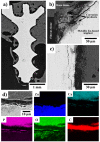
The increasing incidence of trauma in medicine brings with it new demands on the materials used for the surgical treatment of bone fractures. Titanium, its alloys, and steel are used worldwide in the treatment of skeletal injuries. These metallic materials, although inert, are often removed after the injured bone has healed. The second-stage procedure-the removal of the plates and screws-can overwhelm patients and overload healthcare systems. The development of suitable absorbable metallic materials would help us to overcome these issues. In this experimental study, we analyzed an extruded Zn-0.8Mg-0.2Sr (wt.%) alloy on a rabbit model. From this alloy we developed screws which were implanted into the rabbit tibia. After 120, 240, and 360 days, we tested the toxicity at the site of implantation and also within the vital organs: the liver, kidneys, and brain. The results were compared with a control group, implanted with a Ti-based screw and sacrificed after 360 days. The samples were analyzed using X-ray, micro-CT, and a scanning electron microscope. Chemical analysis revealed only small concentrations of zinc, strontium, and magnesium in the liver, kidneys, and brain. Histologically, the alloy was verified to possess very good biocompatibility after 360 days, without any signs of toxicity at the site of implantation. We did not observe raised levels of Sr, Zn, or Mg in any of the vital organs when compared with the Ti group at 360 days. The material was found to slowly degrade in vivo, forming solid corrosion products on its surface.
Keywords: absorbable metals; alloy accumulation; biocompatibility; in vivo; internal organs; magnesium; strontium; systemic reactions; toxicity; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Han H.-S., Loffredo S., Jun I., Edwards J., Kim Y.-C., Seok H.-K., Witte F., Mantovani D., Glyn-Jones S. Current Status and Outlook on the Clinical Translation of Biodegradable Metals. Mater. Today. 2019;23:57–71. doi: 10.1016/j.mattod.2018.05.018. - DOI
-
- Tan L., Yu X., Wan P., Yang K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013;29:503–513. doi: 10.1016/j.jmst.2013.03.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

