The Progress of Intestinal Epithelial Models from Cell Lines to Gut-On-Chip
- PMID: 34948271
- PMCID: PMC8709104
- DOI: 10.3390/ijms222413472
The Progress of Intestinal Epithelial Models from Cell Lines to Gut-On-Chip
Abstract
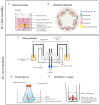
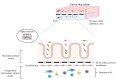
Over the past years, several preclinical in vitro and ex vivo models have been developed that helped to understand some of the critical aspects of intestinal functions in health and disease such as inflammatory bowel disease (IBD). However, the translation to the human in vivo situation remains problematic. The main reason for this is that these approaches fail to fully reflect the multifactorial and complex in vivo environment (e.g., including microbiota, nutrition, and immune response) in the gut system. Although conventional models such as cell lines, Ussing chamber, and the everted sac are still used, increasingly more sophisticated intestinal models have been developed over the past years including organoids, InTESTine™ and microfluidic gut-on-chip. In this review, we gathered the most recent insights on the setup, advantages, limitations, and future perspectives of most frequently used in vitro and ex vivo models to study intestinal physiology and functions in health and disease.
Keywords: ex vivo; gut-on-chip; in vitro; inflammation; intestine; organoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Benet L.Z., Wu C.-Y., Hebert M.F., Wacher V.J. Intestinal drug metabolism and antitransport processes: A potential paradigm shift in oral drug delivery. J. Control Release. 1996;39:139–143. doi: 10.1016/0168-3659(95)00147-6. - DOI
-
- Moore F.A., Moore E.E., Poggetti R.E.N.A.T.O., McAnena O.J., Peterson V.M., Abernathy C.M., Parsons P.E. Gut bacterial translocation via the portal vein: A clinical perspective with major torso trauma. J. Trauma. 1991;31:629–636. doi: 10.1097/00005373-199105000-00006. discussion 636–638. - DOI - PubMed
-
- Ahuja M., Schwartz D., Tandon M., Son A., Zeng M., Swaim W., Eckhaus M., Hoffman V., Cui Y., Xiao B., et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017;25:635–646. doi: 10.1016/j.cmet.2017.02.007. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

