The Nutritional Supplement L-Alpha Glycerylphosphorylcholine Promotes Atherosclerosis
- PMID: 34948275
- PMCID: PMC8708068
- DOI: 10.3390/ijms222413477
The Nutritional Supplement L-Alpha Glycerylphosphorylcholine Promotes Atherosclerosis
Abstract
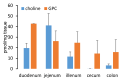
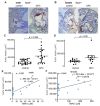
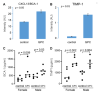
L-alpha glycerylphosphorylcholine (GPC), a nutritional supplement, has been demonstrated to improve neurological function. However, a new study suggests that GPC supplementation increases incident stroke risk thus its potential adverse effects warrant further investigation. Here we show that GPC promotes atherosclerosis in hyperlipidemic Apoe-/- mice. GPC can be metabolized to trimethylamine N-oxide, a pro-atherogenic agent, suggesting a potential molecular mechanism underlying the observed atherosclerosis progression. GPC supplementation shifted the gut microbial community structure, characterized by increased abundance of Parabacteroides, Ruminococcus, and Bacteroides and decreased abundance of Akkermansia, Lactobacillus, and Roseburia, as determined by 16S rRNA gene sequencing. These data are consistent with a reduction in fecal and cecal short chain fatty acids in GPC-fed mice. Additionally, we found that GPC supplementation led to an increased relative abundance of choline trimethylamine lyase (cutC)-encoding bacteria via qPCR. Interrogation of host inflammatory signaling showed that GPC supplementation increased expression of the proinflammatory effectors CXCL13 and TIMP-1 and activated NF-κB and MAPK signaling pathways in human coronary artery endothelial cells. Finally, targeted and untargeted metabolomic analysis of murine plasma revealed additional metabolites associated with GPC supplementation and atherosclerosis. In summary, our results show GPC promotes atherosclerosis through multiple mechanisms and that caution should be applied when using GPC as a nutritional supplement.
Keywords: L-alpha glycerylphosphorylcholine; atherosclerosis; microbiota; trimethylamine; trimethylamine N-oxide.
Conflict of interest statement
Wang and Hazen report being named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Wang and Hazen report having received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, a fully owned subsidiary of Quest Diagnostics, and Procter & Gamble. Hazen is a paid consultant for Procter & Gamble, Zehna Therapeutics, and has received research funds from Proctor & Gamble, Zehna Therapeutics, Pfizer Inc., and Roche Diagnostics. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures











References
-
- Amenta F., Tayebati S.K., Vitali D., Di Tullio M.A. Association with the cholinergic precursor choline alphoscerate and the cholinesterase inhibitor rivastigmine: An approach for enhancing cholinergic neurotransmission. Mech. Ageing Dev. 2006;127:173–179. doi: 10.1016/j.mad.2005.09.017. - DOI - PubMed
-
- Gatti G., Barzaghi N., Acuto G., Abbiati G., Fossati T., Perucca E. A comparative study of free plasma choline levels following intramuscular administration of L-alpha-glycerylphosphorylcholine and citicoline in normal volunteers. Int. J. Clin. Pharmacol. Ther. Toxicol. 1992;30:331–335. - PubMed
-
- USDA Database for the Choline Content of Common Foods. [(accessed on 4 November 2021)];2008 Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Choline/Choln02.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

