Testing Antigens, Antibodies, and Immune Cells in COVID-19 as a Public Health Topic-Experience and Outlines
- PMID: 34948782
- PMCID: PMC8700871
- DOI: 10.3390/ijerph182413173
Testing Antigens, Antibodies, and Immune Cells in COVID-19 as a Public Health Topic-Experience and Outlines
Abstract
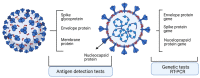
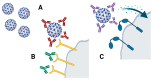
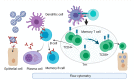
The current COVID-19 pandemic has triggered an accelerated pace in all research domains, including reliable diagnostics methodology. Molecular diagnostics of the virus and its presence in biological samples relies on the RT-PCR method, the most used and validated worldwide. Nonconventional tests with improved parameters that are in the development stages will be presented, such as droplet digital PCR or CRISPR-based assays. These molecular tests were followed by rapid antigen testing along with the development of antibody tests, whether based on ELISA platform or on a chemiluminescent microparticle immunoassay. Less-conventional methods of testing antibodies (e.g., lateral flow immunoassay) are presented as well. Left somewhere in the backstage of COVID-19 research, immune cells and, furthermore, immune memory cells, are gaining the spotlight, more so in the vaccination context. Recently, methodologies using flow-cytometry evaluate circulating immune cells in infected/recovered patients. The appearance of new virus variants has triggered a surge for tests improvement. As the pandemic has entered an ongoing or postvaccination era, all methodologies that are used to monitor public health focus on diagnostic strategies and this review points out where gaps should be filled in both clinical and research settings.
Keywords: SARS-CoV-2; detection; methodology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech).J Clin Virol. 2020 Aug;129:104511. doi: 10.1016/j.jcv.2020.104511. Epub 2020 Jun 15. J Clin Virol. 2020. PMID: 32593133 Free PMC article.
-
Evaluation of performance of two SARS-CoV-2 Rapid IgM-IgG combined antibody tests on capillary whole blood samples from the fingertip.PLoS One. 2020 Sep 17;15(9):e0237694. doi: 10.1371/journal.pone.0237694. eCollection 2020. PLoS One. 2020. PMID: 32941461 Free PMC article.
-
Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies.J Clin Virol. 2020 Jul;128:104413. doi: 10.1016/j.jcv.2020.104413. Epub 2020 May 5. J Clin Virol. 2020. PMID: 32403010 Free PMC article.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
Force-dependent rapid immunoassay of high specificity and sensitivity.Mechanobiol Med. 2024 Mar 21;2(2):100061. doi: 10.1016/j.mbm.2024.100061. eCollection 2024 Jun. Mechanobiol Med. 2024. PMID: 40395855 Free PMC article. Review.
Cited by
-
Systematic Review of Diagnostic Accuracy of DiaSorin Liaison SARS-CoV-2 Antigen Immunoassay.EJIFCC. 2022 Aug 8;33(2):94-104. eCollection 2022 Aug. EJIFCC. 2022. PMID: 36313904 Free PMC article. Review.
-
Clinical challenges of SARS-CoV-2 variants (Review).Exp Ther Med. 2022 Jun;23(6):416. doi: 10.3892/etm.2022.11343. Epub 2022 Apr 28. Exp Ther Med. 2022. PMID: 35601074 Free PMC article. Review.
-
Immunogenicity evaluation after BNT162b2 booster vaccination in healthcare workers.Sci Rep. 2022 Jul 26;12(1):12716. doi: 10.1038/s41598-022-16759-2. Sci Rep. 2022. PMID: 35882871 Free PMC article.
-
Skin Cancer Research Goes Digital: Looking for Biomarkers within the Droplets.J Pers Med. 2022 Jul 13;12(7):1136. doi: 10.3390/jpm12071136. J Pers Med. 2022. PMID: 35887633 Free PMC article. Review.
-
Assessment of Clinical Profile and Treatment Outcome in Vaccinated and Unvaccinated SARS-CoV-2 Infected Patients.Vaccines (Basel). 2022 Jul 15;10(7):1125. doi: 10.3390/vaccines10071125. Vaccines (Basel). 2022. PMID: 35891289 Free PMC article.
References
-
- Neagu M., Calina D., Docea A.O., Constantin C., Filippini T., Vinceti M., Drakoulis N., Poulas K., Taxiarchis Konstantinos Nikolouzakis T.K., Spandidos D.A., et al. Back to basics in COVID-19: Antigens and antibodies—Completing the puzzle. J. Cell. Mol. Med. 2021;25:4523–4533. doi: 10.1111/jcmm.16462. - DOI - PMC - PubMed
-
- Food and Drug Administration EUA Authorized Serology Test Performance. [(accessed on 7 September 2021)]; Available online: https://www.fda.gov/medical-devices/mergency-situations-medical-devices/....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

