Spectrum of Neuroradiologic Findings Associated with Monogenic Interferonopathies
- PMID: 34949589
- PMCID: PMC8757560
- DOI: 10.3174/ajnr.A7362
Spectrum of Neuroradiologic Findings Associated with Monogenic Interferonopathies
Abstract
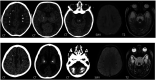
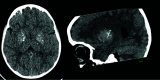
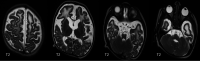
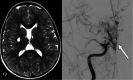
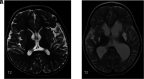
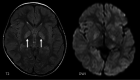
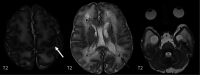
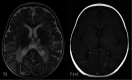
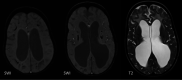
The genetic interferonopathies are a heterogeneous group of disorders thought to be caused by the dysregulated expression of interferons and are now commonly considered in the differential diagnosis of children presenting with recurrent or persistent inflammatory phenotypes. With emerging therapeutic options, recognition of these disorders is increasingly important, and neuroimaging plays a vital role. In this article, we discuss the wide spectrum of neuroradiologic features associated with monogenic interferonopathies by reviewing the literature and illustrate these with cases from our institutions. These cases include intracerebral calcifications, white matter T2 hyperintensities, deep WM cysts, cerebral atrophy, large cerebral artery disease, bilateral striatal necrosis, and masslike lesions. A better understanding of the breadth of the neuroimaging phenotypes in conjunction with clinical and laboratory findings will enable earlier diagnosis and direct therapeutic strategies.
© 2022 by American Journal of Neuroradiology.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
