Cycloserine did not increase depression incidence or severity at standard dosing for multidrug-resistant tuberculosis
- PMID: 34949698
- PMCID: PMC8943271
- DOI: 10.1183/13993003.02511-2021
Cycloserine did not increase depression incidence or severity at standard dosing for multidrug-resistant tuberculosis
Abstract
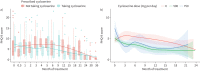
In a longitudinal cohort of MDR-TB patients receiving individualised, DST-based treatment, neither the inclusion of cycloserine in a multidrug regimen nor the dose used (up to 750 mg daily) significantly increased incidence of depression during treatment
Conflict of interest statement
Conflict of interest: J.A. Tornheim reports, during the conduct of the study, salary support from NIH/NIAID (K23AI135102, R21AI122922 and R01AI134430), salary support from the NIH Fogarty International Center (R25TW009340) and salary support from the Johns Hopkins Clinician Scientist Career Development Award; salary support for a study clinician during the first year of the project was provided by the Frederick Mulder Foundation and the Pogge Tong Foundation. C. Rodrigues reports payment for manuscript writing, honoraria for lectures, educational events from Pfizer; honoraria for lectures from Cipla, Glenmark and Novartis; outside the submitted work. All other authors have nothing to disclose.
Figures
References
-
- World Health Organization (WHO) . WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. Geneva, WHO, 2020. - PubMed
-
- van der Galiën R, Boveneind-Vrubleuskaya NV, Peloquin C, et al. Pharmacokinetic modeling, simulation, and development of a limited sampling strategy of cycloserine in patients with multidrug-/extensively drug-resistant tuberculosis. Clin Pharmacokinet 2020; 59: 899–910. doi: 10.1007/s40262-020-00860-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
