Rapid, efficient and activation-neutral gene editing of polyclonal primary human resting CD4+ T cells allows complex functional analyses
- PMID: 34949807
- PMCID: PMC8748193
- DOI: 10.1038/s41592-021-01328-8
Rapid, efficient and activation-neutral gene editing of polyclonal primary human resting CD4+ T cells allows complex functional analyses
Abstract
CD4+ T cells are central mediators of adaptive and innate immune responses and constitute a major reservoir for human immunodeficiency virus (HIV) in vivo. Detailed investigations of resting human CD4+ T cells have been precluded by the absence of efficient approaches for genetic manipulation limiting our understanding of HIV replication and restricting efforts to find a cure. Here we report a method for rapid, efficient, activation-neutral gene editing of resting, polyclonal human CD4+ T cells using optimized cell cultivation and nucleofection conditions of Cas9-guide RNA ribonucleoprotein complexes. Up to six genes, including HIV dependency and restriction factors, were knocked out individually or simultaneously and functionally characterized. Moreover, we demonstrate the knock in of double-stranded DNA donor templates into different endogenous loci, enabling the study of the physiological interplay of cellular and viral components at single-cell resolution. Together, this technique allows improved molecular and functional characterizations of HIV biology and general immune functions in resting CD4+ T cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











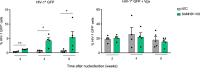
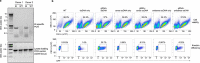


Comment in
-
Editing naive CD4+ T cells.Nat Methods. 2022 Jan;19(1):36-37. doi: 10.1038/s41592-021-01332-y. Nat Methods. 2022. PMID: 34949808 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

