Solvent Additive-Induced Deactivation of the Cu-ZnO(Al2O3)-Catalyzed γ-Butyrolactone Hydrogenolysis: A Rare Deactivation Process
- PMID: 34949902
- PMCID: PMC8689444
- DOI: 10.1021/acs.iecr.1c04080
Solvent Additive-Induced Deactivation of the Cu-ZnO(Al2O3)-Catalyzed γ-Butyrolactone Hydrogenolysis: A Rare Deactivation Process
Erratum in
-
Correction to "Solvent Additive-Induced Deactivation of the Cu-ZnO(Al2O3)-Catalyzed γ-Butyrolactone Hydrogenolysis: A Rare Deactivation Process".Ind Eng Chem Res. 2023 Feb 14;62(8):3833. doi: 10.1021/acs.iecr.3c00343. eCollection 2023 Mar 1. Ind Eng Chem Res. 2023. PMID: 36880852 Free PMC article.
Abstract
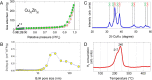
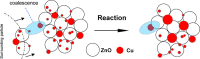
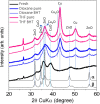
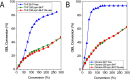
This work reports initial results on the effect of low concentrations (ppm level) of a stabilizing agent (2,6-di-tert-butyl-4-methylphenol, BHT) present in an off-the-shelf solvent on the catalyst performance for the hydrogenolysis of γ-butyrolactone over Cu-ZnO-based catalysts. Tetrahydrofuran (THF) was employed as an alternative solvent in the hydrogenolysis of γ-butyrolactone. It was found that the Cu-ZnO catalyst performance using a reference solvent (1,4-dioxane) was good, meaning that the equilibrium conversion was achieved in 240 min, while a zero conversion was found when employing tetrahydrofuran. The deactivation was studied in more detail, arriving at the preliminary conclusion that one phenomenon seems to play a role: the poisoning effect of a solvent additive present at the ppm level (BHT) that appears to inhibit the reaction completely over a Cu-ZnO catalyst. The BHT effect was also visible over a commercial Cu-ZnO-MgO-Al2O3 catalyst but less severe than that over the Cu-ZnO catalyst. Hence, the commercial catalyst is more tolerant to the solvent additive, probably due to the higher surface area. The study illustrates the importance of solvent choice and purification for applications such as three-phase-catalyzed reactions to achieve optimal performance.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



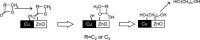

References
-
- Melian-Cabrera I. Catalytic materials: Concepts to understand the pathway to implementation. Ind. Eng. Chem. Res. 2021, 10.1021/acs.iecr.1c02681. - DOI
-
- Froment G. F. The modeling of catalyst deactivation by coke formation. Stud. Surf. Sci. Catal. 1991, 68, 53–83. 10.1016/S0167-2991(08)62620-8. - DOI
-
- Forzatti P.; Lietti L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. 10.1016/S0920-5861(99)00074-7. - DOI
-
- Bartholomew C. H. Mechanisms of catalyst deactivation. Appl. Catal., A 2001, 212, 17–60. 10.1016/S0926-860X(00)00843-7. - DOI
-
- Moulijn J. A.; van Diepen A. E.; Kapteijn F. Catalyst deactivation: Is It predictable? What to do?. Appl. Catal., A 2001, 212, 3–16. 10.1016/S0926-860X(00)00842-5. - DOI
LinkOut - more resources
Full Text Sources
