Immune Checkpoint Inhibitors: The Emerging Cornerstone in Cholangiocarcinoma Therapy?
- PMID: 34950178
- PMCID: PMC8647071
- DOI: 10.1159/000518104
Immune Checkpoint Inhibitors: The Emerging Cornerstone in Cholangiocarcinoma Therapy?
Abstract
Background: Cholangiocarcinoma (CCA) encompasses a heterogeneous group of malignant tumors with dismal prognosis and increasing incidence worldwide. Both late diagnosis due to the lack of early symptoms and the refractory nature of these tumors seriously compromise patients' welfare and outcomes.
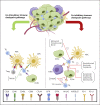
Summary: During the last decade, immunotherapy and, more specifically, modulation of immune checkpoints-mediated signaling pathways have been under the spotlight in the field of oncology, emerging as a potential therapeutic approach for the treatment of several cancers, including CCA. Generally, high expression levels of immune checkpoints in patients with CCA have been associated with worse clinical outcomes, particularly with shorter overall survival and relapse-free survival. Thus, immune checkpoint inhibitors (ICIs), which mainly constitute different monoclonal antibodies, have been developed in order to hamper the immune checkpoint-mediated pathways. Interestingly, chemotherapy may increase the expression of immune checkpoints, while other therapeutic approaches such as ablative and targeted therapies may enhance their antitumor activity. In this sense, several clinical trials evaluated the safety and efficacy of ICIs for CCA, both as a monotherapy and in combination with other ICIs or loco-regional and systemic therapies. Additionally, many other clinical trials are currently ongoing and results are eagerly awaited. Here, we summarize the key aspects of immune checkpoint molecules as prognostic factors and therapeutic targets in CCA, highlighting the most recent advances in the field and future research directions.
Key messages: (1) Effective therapeutic approaches for CCA are urgently needed. (2) Expression levels of immune checkpoints in patients with CCA have been proposed to be related with clinical outcomes. (3) Combination of different ICIs may outperform the efficacy of ICI monotherapy for CCA treatment. (4) Recent studies point toward the combination of ICIs and other common therapies, especially chemotherapy, as a promising strategy for treatment of CCA patients.
Keywords: Cholangiocarcinoma; Clinical trials; Immune checkpoint inhibitors; Prognosis; Therapy.
Copyright © 2021 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Rodrigues PM, Olaizola P, Paiva NA, Olaizola I, Agirre-Lizaso A, Landa A, et al. Pathogenesis of cholangiocarcinoma. Annu Rev Pathol. 2021 Jan 24;16:433–63. - PubMed
-
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019 May;39((Suppl 1)):19–31. - PubMed
-
- Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019 Jul;71((1)):104–14. - PubMed
-
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014 Jun;60((6)):1268–89. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

