Targeted drug delivery strategies for precision medicines
- PMID: 34950512
- PMCID: PMC8691416
- DOI: 10.1038/s41578-020-00269-6
Targeted drug delivery strategies for precision medicines
Abstract
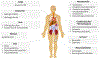
Progress in the field of precision medicine has changed the landscape of cancer therapy. Precision medicine is propelled by technologies that enable molecular profiling, genomic analysis, and optimized drug design to tailor treatments for individual patients. Although precision medicines have resulted in some clinical successes, the use of many potential therapeutics has been hindered by pharmacological issues, including toxicities and drug resistance. Drug delivery materials and approaches have now advanced to a point where they can enable the modulation of a drug's pharmacological parameters without compromising the desired effect on molecular targets. Specifically, they can modulate a drug's pharmacokinetics, stability, absorption, and exposure to tumours and healthy tissues, and facilitate the administration of synergistic drug combinations. This Review highlights recent progress in precision therapeutics and drug delivery, and identifies opportunities for strategies to improve the therapeutic index of cancer drugs, and consequently, clinical outcomes.
Conflict of interest statement
Competing interests statement D.A.H. is a cofounder and officer of with equity interest in Goldilocks Therapeutics, Inc., LipidSense, Inc., and Nirova BioSense, Inc., as well as a member of the scientific advisory boards of Concarlo Holdings, LLC and Nanorobotics, Inc. M.T.M. is a consultant for Synthis, LLC. M.S. has received research funds from Puma Biotechnology, AstraZeneca, Daiichi-Sankio, Immunomedics, Targimmune and Menarini Ricerche, is a cofounder of Medendi.org and is on the advisory board of Menarini Ricerche. N.R. is on the scientific advisory board (SAB) of Chugai, BeiGene, Fortress Biotech, Daiichi-Sankyo, AstraZeneca, F-Prime, Zai Lab, Arvinas, and Array BioPharma; and he is a past SAB member of Millennium-Takeda, Kadmon, Kura Oncology and Araxes. N.R. is also a consultant to Novartis Biomed, Boehringer Ingelheim, Tarveda, Foresite Capital, Array BioPharma, and Revolution Medicines; and in recent years has also consulted with Eli Lilly, Merrimack, Kura Oncology, Araxes, and Kadmon. N.R. owns equity in BeiGene, Zai Lab, Fortress Biotech, Kura Oncology, Araxes, Kadmon and Effector. N.R. collaborates with Plexxikon; he receives research support from Chugai.
Figures

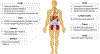

References
-
- Ferguson FM & Gray NS Kinase inhibitors: the road ahead. Nat. Rev. Drug Discov 17, 353 (2018). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
