Household Contact Tracing With Intensified Tuberculosis and Human Immunodeficiency Virus Screening in South Africa: A Cluster-Randomized Trial
- PMID: 34950944
- PMCID: PMC9477445
- DOI: 10.1093/cid/ciab1047
Household Contact Tracing With Intensified Tuberculosis and Human Immunodeficiency Virus Screening in South Africa: A Cluster-Randomized Trial
Abstract
Background: Household contact tracing for tuberculosis (TB) may facilitate diagnosis and access to TB preventive treatment (TPT). We investigated whether household contact tracing and intensive TB/human immunodeficiency virus (HIV) screening would improve TB-free survival.
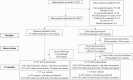
Methods: Household contacts of index TB patients in 2 South African provinces were randomized to home tracing and intensive HIV/TB screening or standard of care (SOC; clinic referral letters). The primary outcome was incident TB or death at 15 months. Secondary outcomes included tuberculin skin test (TST) positivity in children ≤14 years and undiagnosed HIV.
Results: From December 2016 through March 2019, 1032 index patients (4459 contacts) and 1030 (4129 contacts) were randomized to the intervention and SOC arms. Of intervention arm contacts, 3.2% (69 of 2166) had prevalent microbiologically confirmed TB. At 15 months, the cumulative incidence of TB or death did not differ between the intensive screening (93 of 3230, 2.9%) and SOC (80 of 2600, 3.1%) arms (hazard ratio, 0.90; 95% confidence interval [CI], .66-1.24). TST positivity was higher in the intensive screening arm (38 of 845, 4.5%) compared with the SOC arm (15 of 800, 1.9%; odds ratio, 2.25; 95% CI, 1.07-4.72). Undiagnosed HIV was similar between arms (41 of 3185, 1.3% vs 32 of 2543, 1.3%; odds ratio, 1.02; 95% CI, .64-1.64).
Conclusions: Household contact tracing with intensive screening and referral did not reduce incident TB or death. Providing referral letters to household contacts of index patients is an alternative strategy to home visits.
Clinical trials registration: ISRCTN16006202.
Keywords: HIV; diagnosis; randomized controlled trials; screening; tuberculosis.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. N. A. M. reports a grant from Pfizer to their institution for an observational study of pneumonia. E. L. W. reports grant funding to their institution from European and Developing Countries Clinical Trials Partnership (EDCTP) and UK MRC; reports serving as DSMB member for the VietNARMS trial, Trial Steering Committee (TSC) independent member for GUIDES trial steering committee, and DSMB chair for the PREPARE trial. S. G. L. reports support from the Civilian Research and Development Foundation (CRDF) Global and Discovery Fund. J. E. G. reports 2 National Institutes of Health R01 grants. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Grzybowski S, Barnett GD, Styblo K.. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc 1975; 50:90–106. - PubMed
-
- Rieder HL. Contacts of tuberculosis patients in high-incidence countries. Int J Tuberc Lung Dis 2003; 7:S333–6. - PubMed
-
- World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. 2013. Available at: https://www.who.int/tb/tbscreening/en/. Accessed 6 December 2019. - PubMed
-
- Velen K, Shingde RV, Ho J, Fox GJ.. The effectiveness of contact investigation among contacts of tuberculosis patients: a systematic review and meta-analysis. Eur Respir J 2021. Available at: https://erj.ersjournals.com/content/58/6/2100266 - PubMed

