Repurposing of Mycobacterium indicus pranii for the severe form of COVID-19 patients in India: A cohort study
- PMID: 34951021
- PMCID: PMC9015534
- DOI: 10.1002/jmv.27547
Repurposing of Mycobacterium indicus pranii for the severe form of COVID-19 patients in India: A cohort study
Abstract
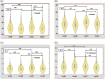
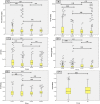
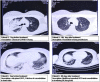
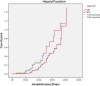
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces the production of proinflammatory cytokines, which results in a cytokine storm, and immune-modulators like Mycobacterium indicus pranii (MIP) might ameliorate coronavirus disease of 2019 (COVID-19) related cytokine storm. Therefore, the present study evaluates whether MIP offers an advantage in the treatment of severe COVID-19 patients infected with SARS-CoV-2. A prospective MIP cohort study was conducted in chest disease hospitals in Srinagar, Jammu and Kashmir, India. In the present prospective, randomized clinical study, critically severe COVID-19 patients were divided into two groups, the MIP group (n = 105) and the best standard treatment (BST) group (n = 210). Procalcitonin, ferritin, high-sensitive C-reactive protein, D-dimer levels, and interleukin levels on 5th-day posttreatment were significantly reduced in the MIP group compared to the BST group. Compared to the BST group, 105 consecutive patients with severe COVID-19 in the MIP group reported early weaning off ventilation, resolution of chest architecture (computed tomography [CT] scan), a significant increase in SpO2 levels, and decreased mortality with a hazard ratio: 0.234 (95% confidence interval: 0.264-2.31) (p = 0.001). MIP restored SpO2 , immune/inflammatory response, normalized lung abnormalities (chest CT scan), and reduced mortality without any serious complications. However, there is a need for placebo-controlled double-blind and controlled clinical trials to confirm the efficacy.
Keywords: COVID-19; Mycobacterium indicus pranii; interleukins; mechanical ventilation; proinflammatory response and oxygen saturation.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures


References
-
- Symptoms page. Accessed February 22, 2021. https://www.cdc.gov/coronavirus/2019-ncov/CDC
-
- Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol. 2014;80(6):509‐514. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

