Biomedical Perspectives of Acute and Chronic Neurological and Neuropsychiatric Sequelae of COVID-19
- PMID: 34951387
- PMCID: PMC9886822
- DOI: 10.2174/1570159X20666211223130228
Biomedical Perspectives of Acute and Chronic Neurological and Neuropsychiatric Sequelae of COVID-19
Abstract
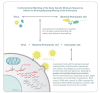
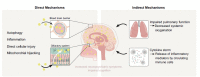
The incidence of infections from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic agent for coronavirus disease 2019 (COVID-19), has dramatically escalated following the initial outbreak in China, in late 2019, resulting in a global pandemic with millions of deaths. Although the majority of infected patients survive, and the rapid advent and deployment of vaccines have afforded increased immunity against SARS-CoV-2, long-term sequelae of SARS-CoV-2 infection have become increasingly recognized. These include, but are not limited to, chronic pulmonary disease, cardiovascular disorders, and proinflammatory-associated neurological dysfunction that may lead to psychological and neurocognitive impairment. A major component of cognitive dysfunction is operationally categorized as "brain fog" which comprises difficulty concentrating, forgetfulness, confusion, depression, and fatigue. Multiple parameters associated with long-term neuropsychiatric sequelae of SARS-CoV-2 infection have been detailed in clinical studies. Empirically elucidated mechanisms associated with the neuropsychiatric manifestations of COVID-19 are by nature complex, but broad-based working models have focused on mitochondrial dysregulation, leading to systemic reductions of metabolic activity and cellular bioenergetics within the CNS structures. Multiple factors underlying the expression of brain fog may facilitate future pathogenic insults, leading to repetitive cycles of viral and bacterial propagation. Interestingly, diverse neurocognitive sequelae associated with COVID-19 are not dissimilar from those observed in other historical pandemics, thereby providing a broad and integrative perspective on potential common mechanisms of CNS dysfunction subsequent to viral infection. Poor mental health status may be reciprocally linked to compromised immune processes and enhanced susceptibility to infection by diverse pathogens. By extrapolation, we contend that COVID-19 may potentiate the severity of neurological/neurocognitive deficits in patients afflicted by well-studied neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease. Accordingly, the prevention, diagnosis, and management of sustained neuropsychiatric manifestations of COVID-19 are pivotal health care directives and provide a compelling rationale for careful monitoring of infected patients, as early mitigation efforts may reduce short- and long-term complications.
Keywords: COVID-19; Central nervous system; SARS-CoV-2; anxiety; brain fog; cognitive impairment; depression; long COVID; microglia; mitochondria; neuroinflammation; neuropsychiatric disease.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures

References
-
- Chou S.H., Beghi E., Helbok R., Moro E., Sampson J., Altamirano V., Mainali S., Bassetti C., Suarez J.I., McNett M., GCS-Neuro COVID Consortium and ENERGY Consortium Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-Neuro COVID consortium and the ENERGY consortium. JAMA Netw. Open. 2021;4(5):e2112131. doi: 10.1001/jamanetworkopen.2021.12131. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

