A new testing platform using fingerstick blood for quantitative antibody response evaluation after SARS-CoV-2 vaccination
- PMID: 34951566
- PMCID: PMC8745372
- DOI: 10.1080/22221751.2021.2023328
A new testing platform using fingerstick blood for quantitative antibody response evaluation after SARS-CoV-2 vaccination
Abstract
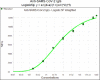
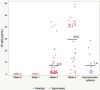
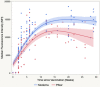
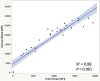
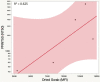
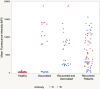
Testing and vaccination have been major components of the strategy for combating the ongoing COVID-19 pandemic. In this study, we have developed a quantitative anti-SARS-CoV-2 spike (S1) IgG antibody assay using a fingerstick dried blood sample. We evaluated the feasibility of using this high-throughput and quantitative anti-SARS-CoV-2 spike (S1) IgG antibody testing assay in vaccinated individuals. Fingerstick blood samples were collected and analyzed from 137 volunteers before and after receiving the Moderna or Pfizer mRNA vaccine. Anti-SARS-CoV-2 S1 IgG antibody could not be detected within the first 7 days after receiving the first vaccine dose, however, the assay reliably detected antibodies from day 14 onwards. In addition, no anti-SARS-CoV-2 nucleocapsid (N) protein IgG antibody was detected in any of the vaccinated or healthy participants, indicating that the anti-SARS-CoV-2 S1 IgG assay is specific for the mRNA vaccine-induced antibodies. The S1 IgG levels detected in fingerstick samples correlated with the levels found in venous blood plasma samples and with the efficacy of venous blood plasma samples in the plaque reduction neutralization test (PRNT). The assay displayed a limit of quantification (LOQ) of 0.59 μg/mL and was found to be linear in the range of 0.51-1000 μg/mL. Finally, its clinical performance displayed a Positive Percent Agreement (PPA) of 100% (95% CI: 0.89-1.00) and a Negative Percent Agreement (NPA) of 100% (95% CI: 0.93-1.00). In summary, the assay described here represents a sensitive, precise, accurate, and simple method for the quantitative detection and monitoring of post-vaccination anti-SARS-CoV-2 spike IgG responses.
Keywords: COVID19; SARS-CoV-2; anti-SARS-CoV-2 igG; fingerstick blood; quantitative immunoassay. SARS-CoV-2 vaccination.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard 2021 https://covid19.who.int.
-
- Liu L, et al. . Potent neutralizing antibodies against multiple epitopes on SARSCoV-2 spike. Nature. 2020;584:450–456. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
