Influence of liver stiffness heterogeneity on staging fibrosis in patients with nonalcoholic fatty liver disease
- PMID: 34951726
- PMCID: PMC9307017
- DOI: 10.1002/hep.32302
Influence of liver stiffness heterogeneity on staging fibrosis in patients with nonalcoholic fatty liver disease
Abstract
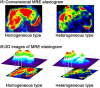
Background and aims: Despite that hepatic fibrosis often affects the liver globally, spatial distribution can be heterogeneous. This study aimed to investigate the effect of liver stiffness (LS) heterogeneity on concordance between MR elastography (MRE)-based fibrosis staging and biopsy staging in patients with NAFLD.
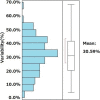
Approach and results: We retrospectively evaluated data from 155 NAFLD patients who underwent liver biopsy and 3 Tesla MRE and undertook a retrospective validation study of 169 NAFLD patients at three hepatology centers. Heterogeneity of stiffness was assessed by measuring the range between minimum and maximum MRE-based LS measurement (LSM). Variability of LSM was defined as the stiffness range divided by the maximum stiffness value. The cohort was divided into two groups (homogenous or heterogeneous), according to whether variability was below or above the average for the training cohort. Based on histopathology and receiver operating characteristic (ROC) analysis, optimum LSM thresholds were determined for MRE-based fibrosis staging of stage 4 (4.43, kPa; AUROC, 0.89) and stage ≥3 (3.93, kPa; AUROC, 0.89). In total, 53 had LSM above the threshold for stage 4. Within this group, 30 had a biopsy stage of <4. In 86.7% of these discordant cases, variability of LSM was classified as heterogeneous. In MRE-based LSM stage ≥3, 88.9% of discordant cases were classified as heterogeneous. Results of the validation cohort were similar to those of the training cohort.
Conclusions: Discordance between biopsy- and MRE-based fibrosis staging is associated with heterogeneity in LSM, as depicted with MRE.
© 2022 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Nothing to report.
Figures

Comment in
-
Letter to the Editor: Influence of liver stiffness heterogeneity on staging fibrosis in patients with nonalcoholic fatty liver disease.Hepatology. 2023 Jul 1;78(1):E18. doi: 10.1097/HEP.0000000000000403. Epub 2023 Apr 15. Hepatology. 2023. PMID: 37054978 No abstract available.
References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Non‐alcoholic fatty liver disease: a spectrum of clinical and pathologic severity. Gastroenterology. 1999;116:1413–9. - PubMed
-
- Day CP, Saksena S. Non‐alcoholic steatohepatitis: definitions and pathogenesis. J Gastroenterol Hepatol. 2002;17(Suppl. 3):S377–84. - PubMed
-
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

