Results of a multicenter phase I/II trial of TCRαβ and CD19-depleted haploidentical hematopoietic stem cell transplantation for adult and pediatric patients
- PMID: 34952929
- PMCID: PMC8702395
- DOI: 10.1038/s41409-021-01551-z
Results of a multicenter phase I/II trial of TCRαβ and CD19-depleted haploidentical hematopoietic stem cell transplantation for adult and pediatric patients
Abstract
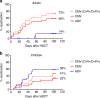
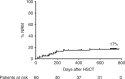
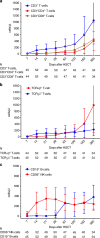
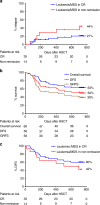
Hematopoietic stem cell transplantation (HSCT) from haploidentical donors is a viable option for patients lacking HLA-matched donors. Here we report the results of a prospective multicenter phase I/II trial of transplantation of TCRαβ and CD19-depleted peripheral blood stem cells from haploidentical family donors after a reduced-intensity conditioning with fludarabine, thiotepa, and melphalan. Thirty pediatric and 30 adult patients with acute leukemia (n = 43), myelodysplastic or myeloproliferative syndrome (n = 6), multiple myeloma (n = 1), solid tumors (n = 6), and non-malignant disorders (n = 4) were enrolled. TCR αβ/CD19-depleted grafts prepared decentrally at six manufacturing sites contained a median of 12.1 × 106 CD34+ cells/kg and 14.2 × 103 TCRαβ+ T-cells/kg. None of the patients developed grade lll/IV acute graft-versus-host disease (GVHD) and only six patients (10%) had grade II acute GVHD. With a median follow-up of 733 days 36/60 patients are alive. The cumulative incidence of non-relapse mortality at day 100, 1 and 2 years after HSCT was 5%, 15%, and 17% for all patients, respectively. Estimated probabilities of overall and disease-free survival at 2 years were 63% and 50%, respectively. Based on these promising results in a high-risk patient cohort, haploidentical HSCT using TCRαβ/CD19-depleted grafts represents a viable treatment option.
© 2021. The Author(s).
Conflict of interest statement
WB: Consultancy and Research Funding—Miltenyi Biotec; Advisory Boards—Celgene, Novartis, Janssen, Gilead. ME: Clinical trial support and advisory boards—Bristol-Myers-Squibb. SM: Data safety monitoring board—Miltenyi, Immunicum; expert panel—Gilead/Kite, Bellicum; speaker’s fee—Novartis, DNA Prime SA, Cellex, Celgene, Kiadis Pharma, Jazz, Miltenyi; travel support—Cellex, Gilead/Kite, MSD, Celgene, Kiadis Pharma, Miltenyi. RM: Consulting or Advisory Role—Bluebird Bio, Bellicum Pharmaceuticals, Novartis; Travel, Accommodations, Expenses—Jazz Pharmaceuticals. DN: Advisory board—Cellectis; speakers bureau—Novartis, Daiichi; manuscript preparation support—Novartis. PGS: Advisory board—Bluebird Bio, not related to the topic of this publication. JK: Shareholder Gadeta and inventor on multiple patents dealing with gdT cell-related topics as well as CAR T isolation strategies; Research Support—Miltenyi Biotec, Novartis. HB: Research support: Bayer, Chugai, Erydel, Miltenyi, Polyphor, Sandoz-Hexal (a Novartis Company), Stage (a Celgene Company), Terumo BCT, Uniqure; honoraria/speakers‘ fees—Chugai, Fresenius, Genzyme, Kiadis, medac, Miltenyi, Novartis, Sandoz-Hexal, Terumo BCT; consultancy and advisory boards—Boehringer-Ingelheim, Celgene, Genzyme, medac, Novartis, Sandoz-Hexal, Stage, Terumo BCT; royalties—medac; stocks—Healthineers. KW: Advisory board—Novartis AG, not related to the topics of this publication. SB and CS are employees of Miltenyi Biotec. SH, SK, and MM are employees of Miltenyi Biomedicine. RH: Speakers’ honoraria—Miltenyi Biotec; co-patent holder of the TcR alpha-beta depletion. AB, DB, JG, PL, AS, MS, VV, MW: no conflict of interest.
Figures


References
-
- Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transpl. 2017;52:811–7. doi: 10.1038/bmt.2017.34. - DOI - PMC - PubMed
-
- Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Cunningham-Rundles S, Dupont B, et al. Transplantation for severe combined immunodeficiency with HLA-A,B,D,DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61:341–8. doi: 10.1182/blood.V61.2.341.341. - DOI - PubMed
-
- Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, et al. Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84:3948–55. doi: 10.1182/blood.V84.11.3948.bloodjournal84113948. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

