Rapid processing and quantitative evaluation of structural brain scans for adaptive multimodal imaging
- PMID: 34953014
- PMCID: PMC8886661
- DOI: 10.1002/hbm.25755
Rapid processing and quantitative evaluation of structural brain scans for adaptive multimodal imaging
Abstract
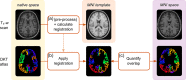
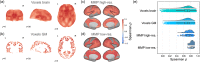
Current neuroimaging acquisition and processing approaches tend to be optimised for quality rather than speed. However, rapid acquisition and processing of neuroimaging data can lead to novel neuroimaging paradigms, such as adaptive acquisition, where rapidly processed data is used to inform subsequent image acquisition steps. Here we first evaluate the impact of several processing steps on the processing time and quality of registration of manually labelled T1 -weighted MRI scans. Subsequently, we apply the selected rapid processing pipeline both to rapidly acquired multicontrast EPImix scans of 95 participants (which include T1 -FLAIR, T2 , T2 *, T2 -FLAIR, DWI and ADC contrasts, acquired in ~1 min), as well as to slower, more standard single-contrast T1 -weighted scans of a subset of 66 participants. We quantify the correspondence between EPImix T1 -FLAIR and single-contrast T1 -weighted scans, using correlations between voxels and regions of interest across participants, measures of within- and between-participant identifiability as well as regional structural covariance networks. Furthermore, we explore the use of EPImix for the rapid construction of morphometric similarity networks. Finally, we quantify the reliability of EPImix-derived data using test-retest scans of 10 participants. Our results demonstrate that quantitative information can be derived from a neuroimaging scan acquired and processed within minutes, which could further be used to implement adaptive multimodal imaging and tailor neuroimaging examinations to individual patients.
Keywords: EPImix; MRI; fingerprinting; identifiability; morphometric similarity; reliability; structural covariance.
© 2021 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

