Evaluation of the Gam-COVID-Vac and vaccine-induced neutralizing response against SARS-CoV-2 lineage P.1 variant in an Argentinean cohort
- PMID: 34953609
- PMCID: PMC8685184
- DOI: 10.1016/j.vaccine.2021.12.027
Evaluation of the Gam-COVID-Vac and vaccine-induced neutralizing response against SARS-CoV-2 lineage P.1 variant in an Argentinean cohort
Abstract
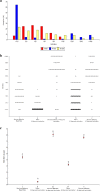
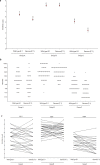
We evaluated humoral immune-response elicited by Sputnik-V by measuring anti-Spike (S) IgG antibodies (Abs) and neutralizing antibodies (NAb) prior to, 14 and 42 days after-vaccination. The safety and disease rates among vaccinated individuals were also evaluated. Since SARS-CoV-2 lineage P.1 is rapidly spreading in Argentina, virus-neutralizing activity of Sputnik-V-elicited and infection-elicited NAb faced to P.1 were also assessed. A total of 285 participants were recruited; all reported good tolerance, without any severe adverse event. Nine COVID-19 cases were confirmed in fully vaccinated individuals and viable P.1 variant was successfully isolated from one of them. At day 42, 99.65% of the individuals had anti-S IgG; however, 23.15% had not detectable NAbs. Significantly higher neutralization potency against WT compared to P.1 (p < 0·001) was observed. Some samples failed to neutralize P.1, mainly among vaccinated-naїve subjects; however, no significant differences were observed among previously infected-vaccinated individuals. Our results corroborated that Sputnik-V is safe and induces an efficient humoral immune response, although not all immunized subjects develop Nabs. Herein, we show for the first time, evidence of infectious SARS-CoV-2 shedding from Sputnik-V fully vaccinated individuals, by the isolation of viable virus from the nasopharyngeal swab of one participant of our study, 139 days after receiving the second dose. Thereby, we provide evidence indicating that the vaccine might avoid severe forms of COVID-19 but does not prevent infection nor prevents transmission from a fully vaccinated individual.
Keywords: COVID-19; Neutralizing antibodies; SARS-CoV-2; SARS-CoV-2 lineage P.1; Sputnik-V; Variants of concern.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Controversy surrounding the Sputnik V vaccine.Respir Med. 2021 Oct;187:106569. doi: 10.1016/j.rmed.2021.106569. Epub 2021 Aug 10. Respir Med. 2021. PMID: 34399368 Free PMC article. Review.
-
Longitudinal Study after Sputnik V Vaccination Shows Durable SARS-CoV-2 Neutralizing Antibodies and Reduced Viral Variant Escape to Neutralization over Time.mBio. 2022 Feb 22;13(1):e0344221. doi: 10.1128/mbio.03442-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073758 Free PMC article.
-
Longitudinal Follow-Up of the Immunity to SARS-CoV-2 in Health Care Workers in Argentina: Persistence of Humoral Response and Neutralizing Capacity after Sputnik V Vaccination.mSphere. 2023 Jun 22;8(3):e0066222. doi: 10.1128/msphere.00662-22. Epub 2023 Apr 18. mSphere. 2023. PMID: 37070983 Free PMC article.
-
Humoral and cellular response of two different vaccines against SARS-CoV-2 in a group of healthcare workers: An observational study.J Immunol Methods. 2024 May;528:113665. doi: 10.1016/j.jim.2024.113665. Epub 2024 Mar 14. J Immunol Methods. 2024. PMID: 38490578
-
An unusual occurrence of autoimmune pancreatitis after gam-Covid-Vac (Sputnik V): A case report and literature review.Br J Clin Pharmacol. 2023 Sep;89(9):2915-2919. doi: 10.1111/bcp.15817. Epub 2023 Jul 1. Br J Clin Pharmacol. 2023. PMID: 37311707 Review.
Cited by
-
Cross-Reactivity of SARS-CoV-2 Nucleocapsid-Binding Antibodies and Its Implication for COVID-19 Serology Tests.Viruses. 2022 Sep 14;14(9):2041. doi: 10.3390/v14092041. Viruses. 2022. PMID: 36146847 Free PMC article.
-
Cuprous oxide nanoparticles incorporated into a polymeric matrix embedded in fabrics to prevent spread of SARS-CoV-2.Int J Pharm. 2023 Apr 5;636:122790. doi: 10.1016/j.ijpharm.2023.122790. Epub 2023 Mar 1. Int J Pharm. 2023. PMID: 36863542 Free PMC article.
-
Controversy surrounding the Sputnik V vaccine.Respir Med. 2021 Oct;187:106569. doi: 10.1016/j.rmed.2021.106569. Epub 2021 Aug 10. Respir Med. 2021. PMID: 34399368 Free PMC article. Review.
-
COVID-19 vaccines: Update of the vaccines in use and under development.Vacunas. 2022 Sep-Dec;23:S88-S102. doi: 10.1016/j.vacun.2022.06.003. Epub 2022 Jun 22. Vacunas. 2022. PMID: 35761987 Free PMC article. Review.
-
A Statistical Synopsis of COVID-19 Components and Descriptive Analysis of Their Socio-Economic and Healthcare Aspects in Bangladesh Perspective.J Environ Public Health. 2023 Feb 13;2023:9738094. doi: 10.1155/2023/9738094. eCollection 2023. J Environ Public Health. 2023. PMID: 36815185 Free PMC article.
References
-
- Logunov D.Y., Dolzhikova I.V., Zubkova O.V., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;96(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. - DOI - PMC - PubMed
-
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. - DOI - PMC - PubMed
-
- Baraniuk C: Covid-19: What do we know about Sputnik V and other Russian vaccines? BMJ 2021;372:n743 doi;10.1136/bmj.n743. - PubMed
-
- Bucci E, Berkhof J, Gillibert A, et al: What do we know about Sputnik V and other Russian vaccines? Rapid Response. BMJ 2021b;372 doi:10.1136/bmj.n743. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

