Vimentin and cytokeratin: Good alone, bad together
- PMID: 34953942
- PMCID: PMC9213573
- DOI: 10.1016/j.semcancer.2021.12.006
Vimentin and cytokeratin: Good alone, bad together
Abstract
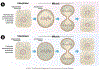
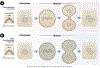
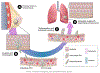
The cytoskeleton plays an integral role in maintaining the integrity of epithelial cells. Epithelial cells primarily employ cytokeratin in their cytoskeleton, whereas mesenchymal cells use vimentin. During the epithelial-mesenchymal transition (EMT), cytokeratin-positive epithelial cells begin to express vimentin. EMT induces stem cell properties and drives metastasis, chemoresistance, and tumor relapse. Most studies of the functions of cytokeratin and vimentin have relied on the use of either epithelial or mesenchymal cell types. However, it is important to understand how these two cytoskeleton intermediate filaments function when co-expressed in cells undergoing EMT. Here, we discuss the individual and shared functions of cytokeratin and vimentin that coalesce during EMT and how alterations in intermediate filament expression influence carcinoma progression.
Keywords: Cancer stem cells; Cytokeratin; Epithelial-mesenchymal transition; Intermediate filaments; Vimentin.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no conflict of interest.
Figures



References
-
- Szeverenyi I. et al. The human intermediate filament database: Comprehensive information on a gene family involved in many human diseases. Hum. Mutat 29, 351–360 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

