Long non-coding RNA ACTA2-AS1 promotes ductular reaction by interacting with the p300/ELK1 complex
- PMID: 34953958
- PMCID: PMC8934273
- DOI: 10.1016/j.jhep.2021.12.014
Long non-coding RNA ACTA2-AS1 promotes ductular reaction by interacting with the p300/ELK1 complex
Abstract
Background & aims: Biliary disease is associated with a proliferative/fibrogenic ductular reaction (DR). p300 is an epigenetic regulator that acetylates lysine 27 on histone 3 (H3K27ac) and is activated during fibrosis. Long non-coding RNAs (lncRNAs) are aberrantly expressed in cholangiopathies, but little is known about how they recruit epigenetic complexes and regulate DR. We investigated epigenetic complexes, including transcription factors (TFs) and lncRNAs, contributing to p300-mediated transcription during fibrosis.
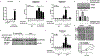
Methods: We evaluated p300 in vivo using tamoxifen-inducible, cholangiocyte-selective, p300 knockout (KO) coupled with bile duct ligation (BDL) and Mdr KO mice treated with SGC-CBP30. Primary cholangiocytes and liver tissue were analyzed for expression of Acta2-as1 lncRNA by qPCR and RNA in situ hybridization. In vitro, we performed RNA-sequencing in human cholangiocytes with a p300 inhibitor. Cholangiocytes were exposed to lipopolysaccharide (LPS) as an injury model. We confirmed formation of a p300/ELK1 complex by immunoprecipitation (IP). RNA IP was used to examine interactions between ACTA2-AS1 and p300. Chromatin IP assays were used to evaluate p300/ELK1 occupancy and p300-mediated H3K27ac. Organoids were generated from ACTA2-AS1-depleted cholangiocytes.
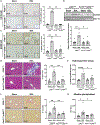
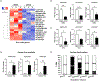
Results: BDL-induced DR and fibrosis were reduced in Krt19-CreERT/p300fl/fl mice. Similarly, Mdr KO mice were protected from DR and fibrosis after SGC-CBP30 treatment. In vitro, depletion of ACTA2-AS1 reduced expression of proliferative/fibrogenic markers, reduced LPS-induced cholangiocyte proliferation, and impaired organoid formation. ACTA2-AS1 regulated transcription by facilitating p300/ELK1 binding to the PDGFB promoter after LPS exposure. Correspondingly, LPS-induced H3K27ac was mediated by p300/ELK1 and was reduced in ACTA2-AS1-depleted cholangiocytes.
Conclusion: Cholangiocyte-selective p300 KO or p300 inhibition attenuate DR/fibrosis in mice. ACTA2-AS1 influences recruitment of p300/ELK1 to specific promoters to drive H3K27ac and epigenetic activation of proliferative/fibrogenic genes. This suggests that cooperation between epigenetic co-activators and lncRNAs facilitates DR/fibrosis in biliary diseases.
Lay summary: We identified a three-part complex containing an RNA molecule, a transcription factor, and an epigenetic enzyme. The complex is active in injured bile duct cells and contributes to activation of genes involved in proliferation and fibrosis.
Keywords: ACTA2-AS1; ELK1; cholangiocyte; ductular reaction; p300.
Copyright © 2021 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest relevant to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

