An mRNA vaccine to prevent genital herpes
- PMID: 34954087
- PMCID: PMC8695322
- DOI: 10.1016/j.trsl.2021.12.006
An mRNA vaccine to prevent genital herpes
Abstract
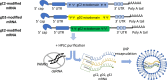
The rapid development of two nucleoside-modified mRNA vaccines that are safe and highly effective against coronavirus disease 2019 has transformed the vaccine field. The mRNA technology has the advantage of accelerated immunogen discovery, induction of robust immune responses, and rapid scale up of manufacturing. Efforts to develop genital herpes vaccines have been ongoing for 8 decades without success. The advent of mRNA technology has the potential to change that narrative. Developing a genital herpes vaccine is a high public health priority. A prophylactic genital herpes vaccine should prevent HSV-1 and HSV-2 genital lesions and infection of dorsal root ganglia, the site of latency. Vaccine immunity should be durable for decades, perhaps with the assistance of booster doses. While these goals have been elusive, new efforts with nucleoside-modified mRNA-lipid nanoparticle vaccines show great promise. We review past approaches to vaccine development that were unsuccessful or partially successful in large phase 3 trials, and describe lessons learned from these trials. We discuss our trivalent mRNA-lipid nanoparticle approach for a prophylactic genital herpes vaccine and the ability of the vaccine to induce higher titers of neutralizing antibodies and more durable CD4+ T follicular helper cell and memory B cell responses than protein-adjuvanted vaccines.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures


References
-
- Lafferty W.E., Downey L, Celum C, et al. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. J Infect Dis. 2000;181:1454–1457. - PubMed
-
- Tuokko H., Bloigu R., Hukkanen V. Herpes simplex virus type 1 genital herpes in young women: current trend in Northern Finland. Sex Transm Infect. 2014;90:160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

